Alzheimer’s disease (AD) is a neurodegenerative disease characterized by memory loss and other cognitive impairments such as thinking and behavior. AD is a type of dementia and can be very debilitating on the body of the patient and also significantly affect the relationships that one has with their family and friends.
Alright, so you’re on your computer or you’re watching Netflix at home and your stomach starts to rumble and you get up to get a snack-what do you grab? Do you go for those veggies sitting in your fridge, decide to make a salad, or say “to heck with being healthy, lets eat some cake and order in Dominos.” While I love ‘junk food’ just as much as the next person, I’m not sure I’ll be reaching for that dessert as much anymore after finding out that type 2 diabetes is a major risk factor for Alzheimer’s disease. Now, just to be clear, I’m not saying that eating a continuous high-fat diet will cause you to have symptoms characteristic of dementia or AD, but I am saying that we, as a society, should start paying closer attention to what we are eating.

Wait, so you’re telling me that there’s a link between my diet, developing type 2 diabetes, and getting AD? How in the world is this possible? I’m glad you asked! There are mechanisms found at the molecular level that help bring this association to light, specifically in regards to insulin signaling, and inflammation.
Insulin Resistance Signaling
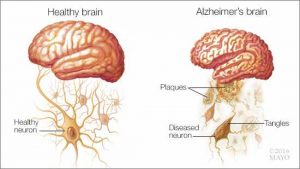
As you’re eating whatever snack you decided on earlier, the food is being broken down into smaller molecules (i.e., glucose) that can be used for energy and metabolism. When there becomes an increased amount of glucose in the body, a hormone, namely insulin, is release. This key hormone helps to breakdown glucose so that the body can use it efficiently and effectively. However, in type 2 diabetes, this entire process is inhibited in some way, thus producing insulin resistance, characterized by having insulin but lacking the proper signaling in the brain. This resistance to insulin can have some negative affects on the brain and body overall. This impaired insulin signaling happening in the brain has been detected in a post-mortem analysis of the brain (the only way to actually ‘diagnose’ AD, as well) in the hippocampus region.
Inflammation
This is an important part of the type 2 diabetes-AD story. Inflammation in type 2 diabetes is mediated by macrophages in adipose tissue that also intersects with ER stress and thus can also be linked to insulin resistance through the TNF-alpha pathway. However, in the brain, this notion of inflammation is mediated by microglia cells that impairs synaptic functioning, also plays a role in ER stress, and is associated with insulin resistance signaling in the brain (specifically with the insulin receptors). This problem of insulin resistance may be due to the fact that there is activation of PKR (protein kinase R) by the TNF-alpha signaling, which would further lead to a potential inhibition of insulin receptor substrate (IRS) in the PI3K/Akt pathway (more on this in a bit).
The PI3K/Akt pathway is important as it is involved in cell growth and proliferation. But what exactly is going wrong in this pathway that leads to cognitive decline and AD? This pathway is overactive in the AD brain and is not being properly shut off. Let’s take a closer look:
This pathway is activated by insulin and leads to a phosphorylation cascade of events that activate a number of different enzymes in the cell. When this pathway is overactive, it leads to insulin resistance in the brain, similar to what is seen in type 2 diabetes due to unhealthy eating habits. Furthermore, when this pathway is constantly being turned on, our bodies try to compensate for this constant activation by decreasing the number of insulin receptors. This ultimately means that there are less receptors for insulin to bind to, and thus less activation of the pathway. However, this essentially leads to insulin resistance in the brain and can be fatal, as mentioned above.
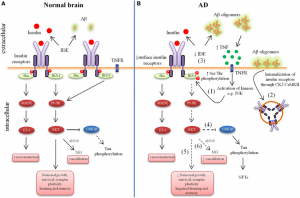
Figure 2. Schematic of the PI3K/Akt pathway.
This over activation can also lead to hyperphosphorylation of the tau protein in the brain-once these start sticking together too much, they create what is called neurofibrillary tangles. This excess phosphorylated tau protein and NFTs can lead to formation of amyloid-beta plaques, which are all highly characteristic of AD.
For more info on tau protein, NFTs, and amyloid-beta plaques: https://www.nia.nih.gov/health/what-happens-brain-alzheimers-disease
Finally, it has also been shown that the hormone leptin plays a major role in helping link type 2 diabetes and AD together. Leptin activates the PI3K/Akt, pathways, which aid in neuronal survival and thus decreasing tau protein phosphorylation and amyloid beta plaques. Thus, if leptin is mutated or inhibited in some way, these pathways aren’t activated, and thus leads to neurodegenerative disease, such as Alzheimer’s. Leptin receptor activation has also been shown to improve the impaired insulin growth factor cell signaling pathway (insulin receptors), thus to normalize cell repair and other processes. This would help to generate normal insulin receptor activation and thus decrease the symptoms seen with AD.
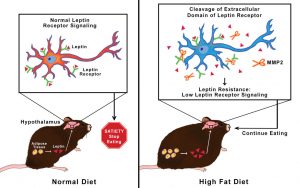
Figure 3. A research study that examined the role of a high-fat diet and the leptin pathway. They found that the mice given the high-fat diet produced the MMP2 enzyme that cut leptin receptors in the brain (hypothalamus), which thus prevents leptin from binding to its receptors. This helps to put together why your brain doesn’t tell your stomach to stop eating because you are full.
So, what? Is there a cure? What’s the big picture anyway?
While there is no cure for AD, it is all about helping the person cope with the symptoms, help relieve the pain and suffering, as well as provide hope for not only the patient but also the family. As you can see, it is quite evident that type 2 diabetes and AD are major risk factors for each other, and it is important to maintain a healthy diet and try to exercise regularly. It may be difficult at times to resist those McDonald’s fries, but a non high-fat diet could go a long way in preventing AD. There is still SO much unknown about Alzheimer’s, but that’s the beauty of science. With advancements in technology and understanding the pathophysiology behind AD and type 2 diabetes, there is always hope for more research to be done in the attempt to finding a cure.
Image 1 from: https://emedmultispecialtygroup.com/2018/03/20/alzheimers-disease-symptoms-care/
Image 2 from: https://newsnetwork.mayoclinic.org/discussion/mayo-clinic-q-and-a-identifying-alzheimers-in-its-earliest-stages/
Image 3 from: https://www.researchgate.net/figure/Aberrant-brain-insulin-signaling-in-Alzheimers-Disease-AD-Schematic-outline-of_fig1_279729041
Image 4 from: https://medicalxpress.com/news/2018-08-destructive-mechanism-blocks-brain.html