INSTILL A LOVE FOR LEARNING
Time is the most valuable thing we have, and as I approach the final week of my undergraduate studies in Neuroscience and Psychology at Concordia. I’ve realized that time moves fast. I’ve always been deeply interested in the brain, which is arguably the most vital organ in our bodies. This curiosity has driven both my academic and personal growth. My experiences this semester have only reinforced my commitment to understanding the complexities of the human mind. It is important to be passionate about learning because it curates a lasting dedication to learning. I believe if you’re passionate and driven, you can accomplish anything.
DEVELOP AN UNDERSTANDING: DISCIPLINARY, INTERDISCIPLINARY, AND INTERCULTURAL PERSPECTIVES
This neurochemistry course has been a great class in concluding my neuroscience degree, Neuroscience gives the opportunity to understand disciplinary, interdisciplinary, and intercultural perspectives. Neurochemistry goes beyond understanding the biochemical signals of neural networks by application to real-world problems, I’ve learned to appreciate how these scientific discoveries relate with intercultural narratives and ethical debates. I have had the opportunity within my five years here to engage with diverse viewpoints. Whether that is exploring genetic influences on behavior or examining the societal implications of mental health research. The articles assigned over the semester have opened my mind to new possibilities. I feel as if I have had the opportunity to learn beyond the textbooks and lectures, which contributed to my learning
Class discussion has proven to be invaluable with the ability to listen and converse with others. Learning is not confined to textbooks. It thrives on curiosity and the willingness to see connections across different fields and cultures.
CULTIVATE: CULTURAL, ETHICAL, PHYSICAL AND SPIRITUAL SELF UNDERSTANDING
Learning at a liberal arts institution like Concordia has meant more than just absorbing information. it has been about nurturing a cultural, ethical, physical, and spiritual self. Concordia gives the opportunity to speak and be heard. My journey in this class has pushed me to reflect on who I am and how I relate to the world around me. As well as the ethical responsibilities that come with scientific discovery. We have covered not only scientific deficits, but social deficits as well that contribute to the well-being of society. This reflective process has allowed me to develop a deeper self-understanding, encouraging me within my academic pursuits.
Cultivating education means engaging in a process that is as much about self-understanding as it is about acquiring knowledge. For me, this kind of learning is rooted in curiosity, resilience, and reflection.
Curiosity
Curiosity pushed me to look beyond the biochemical signals of neural networks and explore how these scientific insights relate to broader cultural narratives and ethical debates. Curiosity sparks meaning which drives me to pursue a deeper meaning behind the data and science.
Resilience
There is value in resilience, much like how the brain has the ability to adapt to its environment, I have learned throughout my stay at Concordia that I have to learn to adjust my approach when confronted with complex problems and changing ideas. Resilience has become the driving force behind my efforts to understand intricate neural signals, diverse cultural narratives, and engage in ethical debates. It’s not just about bouncing back from setbacks; it’s about using each challenge as an opportunity to deepen my understanding and build my learning process. .
Reflection
Lifelong learning is a commitment. Every challenge is an opportunity to grow. It is important to question ideas and not just accept them as they are. Through reflection I can learn about the subject that is at hand, but also about myself and what I need to grow. In reflection I am evolving and changing my learning in a positive way.
DEVELOP FOUNDATIONAL SKILLS AND TRANSFERABLE INTELLUCTUAL CAPACITIES
The skills and knowledge gained in this class are important not only for my academic progress but, also for my future career as a neuroscience and psychology professional. Critical thinking, problem solving, and interdisciplinary communication has been utilized contributing to my learning and studying habits.
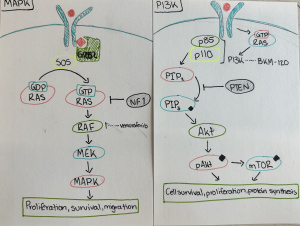
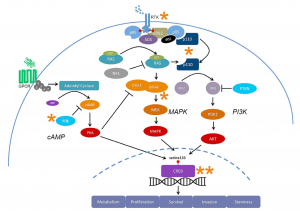
I have had the opportunity to learn through hands-on experiences and rigorous course content. Which have enabled me to develop a suite of foundational skills and knowledge that requires critical thinking, problem solving and interdisciplinary communication. These have been key skills that this course has taught me as I learned to analyze complex neuro-chemical signaling and interpret research papers. This experience encouraged me to ask critical questions and connect specific neuro-chemical findings with broader behavioral contexts.
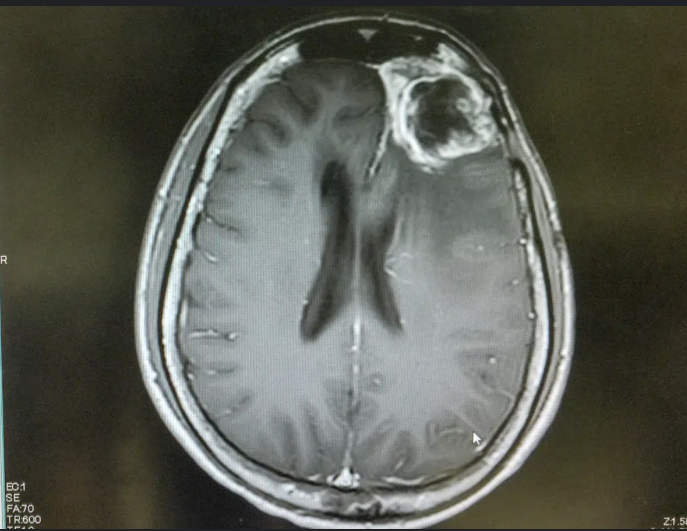
These foundational skills are not confined to the classroom. They transfer to real world contexts, such as disruption in cellular signaling can lead to a serious disease such as cancer or conditions like autism. It provides an opportunity to appreciate the significance of analyzing issues at the molecular level.
ENCOURAGE RESPONSIBLE PARTICIPATION IN THE WORLD
In sum, this semester has not only expanded my intellectual capacities but also reinforced the importance of viewing every challenge as an opportunity to integrate multiple viewpoints. The integration of diverse disciplines has taught me that every academic challenge is an opportunity
Whether it was researching ethical dilemmas in neuro-chemical research or understanding the cultural implications science has. I learned that true education empowers us to take meaningful action in the world. As I move forward in my career, I carry with me the conviction that my knowledge and skills are tools for creating positive change, fostering collaboration, and addressing societal challenges with empathy and rigor.
In reflecting on all these experiences, I realize that the journey here at Concordia is much more than an accumulation of facts—it’s about igniting a deep-seated love for learning that fuels both personal growth and responsible global engagement. My studies in neurochemistry have not only equipped me with technical expertise but have also enriched my understanding of the human condition, preparing me to explore, question, and innovate in ways that honor both science and humanity.



:max_bytes(150000):strip_icc()/government-job-profile-direct-support-professional-1669627-final-24f90e7cdcb841c3b2954fa4997613dd.png)