It hits me during unexpected moments that I’ve grown exponentially during my time at Concordia. It’s been a slow process, and at times unpleasant, but the classes and experiences have pushed me outside of my comfort zone in so many ways, both personally and academically. My science and journalism classes pushed me to do things I never thought I’d do, such as knowing the vast amount of complex topics I’ve learned about and conducting over 15 interviews for journalism classes. I was scared to do it all, and my high school self would’ve recoiled if I had known what I was going to do in college, but I’m glad I did it. It doesn’t seem scary anymore.
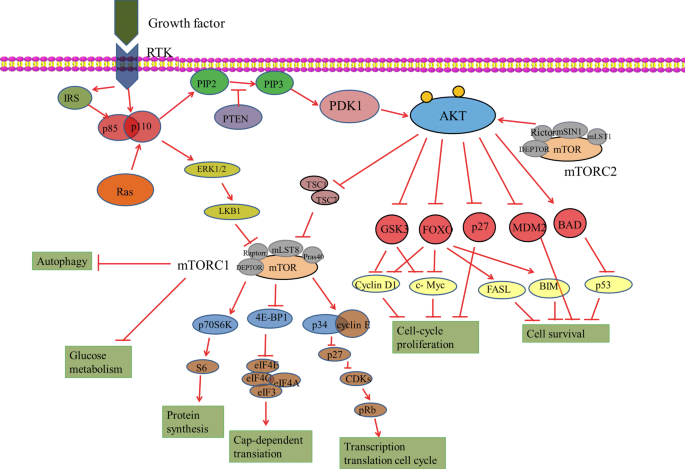
This class certainly pushed me academically. The articles were difficult to understand, and blog posts seemed daunting, but I persevered and grew stronger for it. I now have a greater understanding of the real-world applications of signaling pathways and can bring this knowledge into my future career.
What I’ve Learned This Semester
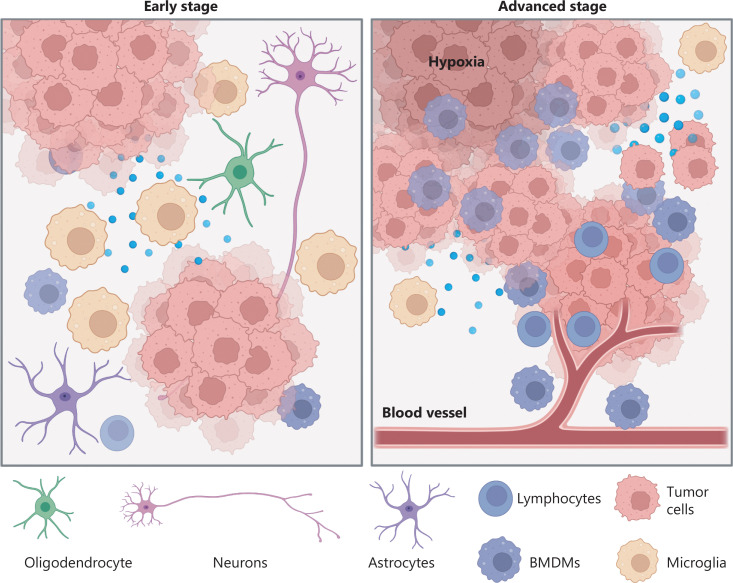
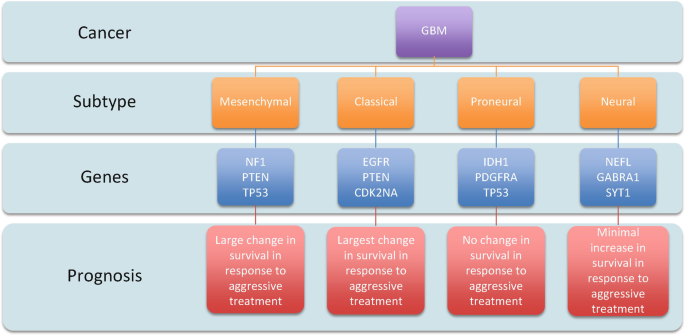
I learned about a variety of topics through this class that may impact my future. We talked about glioblastoma, neurodegeneration, obesity, and Autism, all of which I could encounter in my daily life. Having an understanding of the science brought greater empathy for people experiencing them. I also have greater respect for researchers that are trying to cure cancer or prevent neurodegeneration. I have a greater appreciation for the level of complexity that is happening in the body during these conditions. Before this class, admitedly I didn’t understand why we weren’t further along in our knowledge of cures, but I now see that one aspect of the body will influence a wide range of other parts of the body, and it is not that simple.
My Future Goals
I wish I had a detailed plan for my future and what I want to do with my life, but truthfully, it looks more like a vague idea. I want my career to be meaningful and make an impact on other people. However that will look, I’m not sure yet, but I trust myself to find it. I’m confident that the topics and skills I’ve worked on in this course will help set me up for success. This course strengthened my communication skills, both verbally and in writing, as well as my analytical skills. Both of those areas are crucial for many careers. Even in a person-focused career outside of research, I would need to use analytical skills to solve problems and figure out the best solution.
What does learning at a liberal arts institution mean to you?
A liberal arts degree is about learning from interdisciplinary fields and appreciating the knowledge that other fields have to offer. For instance, though I’m not a history student, I appreciate the knowledge I know from history classes because I can use those use those topics to understand current events and political issues. Math is used daily for simple tasks such as counting how many tables you need for an event. Liberal arts help prepare students to critically engage with issues that are outside of their majors and study, and have a well-rounded education. It’s with these skills that we can succeed amidst challenges.
Skills Gained This Semester
This semester improved communication, analysis, storytelling, and perseverance. I’ve mentioned communication and analysis earlier in this post, but it was really emphasized this semester. I’ve read quite a few scientific papers previously, but this class helped me break down papers and work through them slowly so I understand them. Previously, I would highlight topics and usually forget everything days after reading it, but this class helped teach me to take more time with paper and annotate it so it sticks with me better.
Though I’ve had previous work on written communication through journalism, communicating science was more challenging than writing about campus events. Science communication is it’s own beast, and I’ve had some practice, but it pales in comparison to the amount of science communication that occurs in this class. Taking complex topics and making them into a story is hard, but this class provided the tools to undergo this.
This semester I also worked on perserverence. This was by far my busiest semester. I struggled to find the time to get everything done, and it took a lot of self-work to get to a place where I felt I could manage it. This class was a decent amount of time outside of class, so I’m proud of myself for pushing through and finding stress-relievers to push through a semester that was a shaky juggle of work, school, and personal life.
Using Multidisciplinary Perspectives
This class combined multiple perspectives to analyze problems, ranging from chemistry and neuroscience to sociology and healthcare. When we limit ourselves to using one perspective, we’re doing ourselves a disservice. Other perspectives add so much to any conversation, including scientific conversations, that it’s crucial we integrate the human-aspect of science, such as how these topics are actually impacting society. We can learn a lot from other fields and vice versa. The world is better off with a system where we share out knowledge and build off it, rather than limiting ourselves to one specific lens.
In this class, we didn’t just look at the science behind diabetes, we also discussed the psychology, social aspects, finances, insurance, and healthcare sides of diabetes. It made our conversations more full and impactful.
Conclusion
I’m really happy with the time I’ve spent at Concordia. I’m grateful for the opportunities and the experiences I’ve had here. They’ve helped me grow as a person, and I feel capable to chase my dreams. I’m grateful for the challenging aspects of this course for pushing me out of my comfort zone. I have a bigger toolset for the future because of this course and my liberal arts education. I can approach issues with a multidisciplinary perspective. I’m excited to take on the world, but for now, I’ll appreciate the time I have left on campus and continue to grow as much as I can.





 Abstract by Alisha Debleye depicting the tumorous and dangerous growth in the human brain that can sometimes go untreated or undiagnosed.
Abstract by Alisha Debleye depicting the tumorous and dangerous growth in the human brain that can sometimes go untreated or undiagnosed.