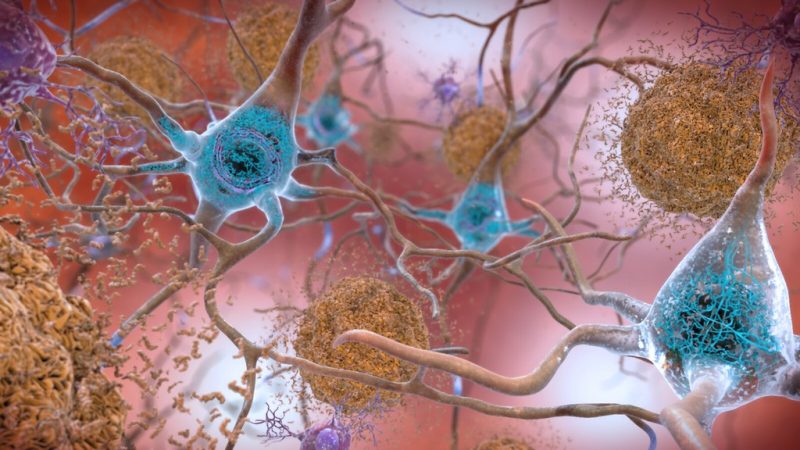
When it comes to common illnesses in the US, diabetes and Alzhiemer’s disease are often quick to make the list. Alzhiemer’s is a type of brain disorder that afflicts an estimated 5.8 million Americans each year, and type 2 diabetes is a metabolic disease that affects a whopping 27-28 million people living within the States. Despite these figures, however, many would be surprised to learn that these seemingly separate diseases share a surprising link. Alzhiemer’s associated with an increased risk of developing type 2 diabetes (and vice versa). Not only that, but in recent years, scientists have found that a hormone whose role is often only heard of in diabetes – the infamous insulin – plays a major part in the brain as well. So how exactly are these pathologies that affect different parts of the body related, and can Alzhiemer’s produce symptoms that could essentially be labeled as another type of diabetes? Keep reading to find out!
Alzheimer’s Disease – Let’s Recall the Basics

Sadly, we all know the story about a grandpa who can’t remember his son’s name, or a grandma who keeps forgetting where she put the keys. Alzheimer’s, or AD for short, is a neurodegenerative disorder (one that damages the nervous system) that leads to memory loss and inability to form new memories. Currently, the condition cannot be reversed or slowed by any existing medicine. Other symptoms might include:
- Repeating statements
- Forgetting scheduled events
- Trouble talking, seemingly lost in speech
- Getting lost, even at home
- Misplacing items consistently
For a full list of symptoms and what you can do for someone experiencing AD, you can visit the Mayo Clinic page, (here)
The key to understanding this terrible disease, and maybe even finding new treatments, lies in the science. It’s commonly known that memory loss and some form of dementia is common with aging, yet we don’t know why. A fairly recent discovery poses that something called an amyloid-beta oligomer (ABO), a type of protein plaque, is found in clumps inside an AD brain, and might contribute to damage associated with AD. Here’s a basic rundown of ABOs in relation to damage:
- Normally function to promote healthy brain growth as proteins called AB40
- Formed by enzymes called secretases
- With age, mutations occur in genes that lead to creation of abnormal AB42 proteins
- Secretases are naturally modified with age, so more AB42 is created
- AB42 clumps on neurons (brain cells) to form ABO plaques
- The brain cannot “clean up” such high amounts of ABOs quickly enough
- ABO plaques trigger brain cells, called microglia, which try to destroy plaques with toxins…
- But in doing so kill neurons and trigger inflammation!
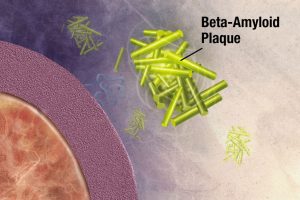
The ABO hypothesis is only one possible pathway that can accelerate damage. Another involves a brain protein, called Tau. In this pathway, Tau proteins are irregularly modified by enzymes, so they contain extra phosphate groups, and form tangles to complement plaques. Quite literally, AD brains are “dirty,” but human brains lack the means to properly clean them up! But how do enzymes go out of whack in the first place?
The Not-So-Sweet Facts About Type 2 Diabetes

Before trying to answer that question, let’s look at a disease that’s seemingly has nothing to do with AD – type 2 diabetes (or T2D). Unlike Alzhiemer’s, T2D has some attributed causes, most linked to poor exercise habits and excessive sugar intake. Notable symptoms include:
- Increased thirst and urination
- Hunger
- Weight loss
- Fatigue
- Trouble healing
For a full list of symptoms, risk factors, and tips for prevention, the Mayo Clinic page is available (here)
T2D has many implications, but in relation with dementia we must focus on how it impacts a specific hormone – insulin. Just like AD, an understanding starts with – you guessed it – science. When you eat a meal, your blood sugar goes up. In response, your pancreas will release insulin to signal all your body cells to take up this extra sugar, or glucose, from your bloodstream. But when there is consistently excessive sugar intake and a lack of physical activity (which normally stimulates the use of glucose), the body might become resistant to its own insulin, or it might produce less, for reasons we’ll explore later. This means the body is less able to store glucose and use it, producing the metabolic symptoms above.
In With Insulin – Bridging the Gap Between AD and T2D
Is insulin the missing link? We can try to bridge the gap between AD and T2D using what we know about insulin resistance. A skim of this review paper offers brilliant insight on exactly how insulin links a brain disorder with a metabolic disorder. To summarize its objective in three statements:
- T2D has long been recognized as an underlying risk factor for developing T2D, and vice versa. Both conditions affect millions of people worldwide, and both social and financial implications are likely, as there is currently no successful means of treatment.
- Understanding the key molecular mechanisms that link the two pathologies together are crucial to the development of potential treatments, but to this day are still under study.
- Therefore, understanding existing research on mammalian models and identifying molecular-level mechanisms of pathogenesis in both diseases is crucial to both medicine and the scientific community.
Sounds complicated? Well it is, but we can start by learning how insulin functions in the brain. Insulin receptors, as mentioned above, are found nearly everywhere in the body, and the brain is no exception. Small amounts are even synthesized in the brain, in (surprise!) the areas important for forming and storing memories, such as the hippocampus. Aside from its metabolic use, insulin signalling serves to protect brain cells, maintain and balance neuron growth, and promote synaptic plasticity (a fancy word for how neurons change to allow for memory formation), via a variety of chemical cascades. An overview of the molecular insulin signalling pathway is visualized in the video below:
Or if you’d rather read:
- Insulin binds to an insulin receptor.
- A phosphate group is added to an amino acid called tyrosine, located on the receptor.
- The receptor proceeds to add phosphate groups to tyrosine on Insulin Receptor Substrate (or IRS proteins – not the Revenue Service).
- Adding a phosphate to tyrosine is like an “on switch,” and the IRS protein team goes on to act on multiple pathways that can trigger changes in gene expression and thus brain changes listed above.
- IRS action can be “switched off” when a different amino acid on IRS – serine – gets a phosphate. This stops insulin signalling.
So T2D leads to insulin resistance, and less response to insulin means the hormone can’t do its work in the brain. T2D causes alzheimer’s, right? Though that might be part of the equation, this becomes a classic example of the chicken and the egg – since both diseases increase the likelihood of developing each other, which one comes first? Luckily, there are several proposed pathways that might aid in our understanding:
Inflammation: Remember those microglia mentioned before? AD is characterized by chronic, low levels of inflammation in the brain, likely due to microglial response to ABO plaques. Likewise, in T2D and obesity, accumulation of adipose (fat) tissue promotes inflammation. When there’s inflammation, immune cells release chemicals called cytokines (primarily a type called TNF-alpha). Not only do these promote more inflammation when they bind with cytokine receptors, but they can also shut off insulin signalling by activating the “serine off switch.” In summary, inflammation brought about by both AD and T2D leads to cytokine release, which can promote insulin resistance, which exacerbates both metabolic symptoms and problems in brain function.
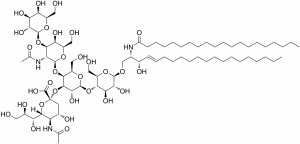
Gangliosides: This funny-sounding word is the name for a molecule (the big one shown here) normally found in cell membranes, notably in neurons. Gangliosides are located in regions of membrane called “microdomains” (quite literally, mini domains). A ganglioside called GM3 is elevated in diabetes, and has been found to disrupt the interaction between insulin receptors and IRS and promote the cytokine-induced resistance described above. Another ganglioside, GM1, decreases GM3 metabolism and can also build up in membranes. Interestingly, GM1 promotes the aggregation of ABOs seen in AD, so more of it leads to more plaque build up, which triggers more inflammation, and the vicious cycle continues! This seemingly simple molecule offers another link between T2D and AD. The only gaping hole is – we don’t currently know why gangliosides become elevated in the first place in T2D.
mTOR: mTOR is a protein normally responsible for cell growth control and regulation. mTOR is a complex topic, and it can be hard to discuss its function without entering the weeds of scientific papers. To oversimplify things a bit, if mTOR is incorrectly activated at any stage of development, developmental and memory problems can occur in the brain. It’s thought that too much mTOR activation leads to insulin resistance by, again, promoting the infamous activation of the “serine off switch” on IRS, by interacting with another protein called S61K. And alas, AD patients are often found to have higher amounts of mTOR! Then again, we don’t know why mTOR goes crazy in the first place. Nonetheless, another link, another connection.
Hope for the Future

After exploring the complex links that exist between these two terrible diseases, is it even possible to try and come up with new treatments, whether for diabetes or Alzhiemer’s? Science is still a long ways from finding an end-all-be-all solution to cognitive decline and insulin resistance, but some of the pathways above offer potential (and quite unexpected) means of therapy:
- Insulin – Starting with the hormone itself. Not only is it used to treat T2D, but administering insulin has been found to boost cognitive effects in previous studies
- Immunosuppresors – Though not ideal, immunosuppressors can reduce immune cell and potentially microglial activity, which could mean less cytokines and inflammation
- Ganglioside targeting drugs – Less gangliosides means less plaque formation, less inflammation, and so on. Studies have found better cognition after using drugs that decrease levels of GM1
- Methods of mTOR inhibition – Drugs that inhibit mTOR could decrease its effects, boosting cognitive performance.
One target that is worth taking note of is PtP1B, as it is thought to be involved in both AD and T2D. Protein tyrosine phosphatase 1B is an enzyme that is normally involved in regulating insulin signalling. It decreases insulin signaling by removing the phosphate “on switch” from tyrosine on both the insulin receptor itself and from our friend, the IRS protein. PtP1B also decreases signalling from a molecule called leptin, which is quite similar in function to insulin in its function within the brain, and also promotes the metabolism of harmful ABOs. To make things worse, PtP1B also inhibits brain derived neurotrophic factor (BDNF), a chemical important for making new neuronal connections and learning. It’s no wonder PtP1B is found at increased levels in Alzhiemer’s patients! This information has led scientists to look at ways to decrease PtP1B activity as modes of treatment.
Getting back to the nagging question, is Alzhiemer’s really another form of diabetes? To keep it short, we don’t really know. But advancements in the scientific and medical communities can be made by understanding how both diseases play into each other. It may be a while before we see treatments that can slow, or even halt, the progression of both types of diseases all together. But like all things in science, great discovery must start with even greater inquiry.
For more information about alzhiemer’s, you can check out this organization here