What are Cannibinoids?
Cannabinoids exist naturally in the body as N-arachidnonyl-ethanolamine (AEA) and 2-arachidonylglycerol (2-AG). They bind to two different types of cannabinoid receptors CB1R and CB2R. AEA and 2-AG are produced by the body in response to increases in the concentrations of calcium in the cell. These molecules are different from other neurotransmitters in the brain because they are unable to diffuse freely in the brain and have to rely concentration gradients or transport systems. Cannabinoids can also come from outside the body in the form of cannabis or synthetic cannabinoids who have similar effects on the body as endocannabinoids.
https://www.ncbi.nlm.nih.gov/pubmed/29533978
Endocannabinoid Signaling

The image above gives a good view of cannabinoid signaling. The cannabinoid receptor is a G-protein coupled receptor and when it is bound to the G protein, which in this case is an inhibitor or can regulate enzymes and channels. As seen in the graphic it inhibits adenylyl cyclase and its downstream effects, activates ERK which slows the cell cycle, and through the actions of ceramide it can inhibit AKT and mTOR which have a role in cell death. This particular graphic is focused on the role of endocannabinoids in cancer patients so this is where the halting of tumor progression via the actions of RhoA and the inhibition of the cell cycle resulting from the activation of ERK is important in the disease progression and treatment through cannabinoids.
https://www.cell.com/trends/pharmacological-sciences/fulltext/S0165-6147(13)00044-8
Cannabinoids for Disease Treatment
There are currently three canabionoid derived medications that are approved by the FDA for disease treatment. Epidiolex is a drug that has been approved for the treatment of seizures that are related to Lennox-Gastaut Syndrome and Dravet Syndrome. There is also a wealth of anecdotal evidence about the efficacy of people using cannabis derived products for the treatment of epilepsy. Other drugs have been approved to treat weight loss that is associated with anorexia and with AIDS. Research is also ongoing about the use of these drugs to treat weight loss in cancer patients.
https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd
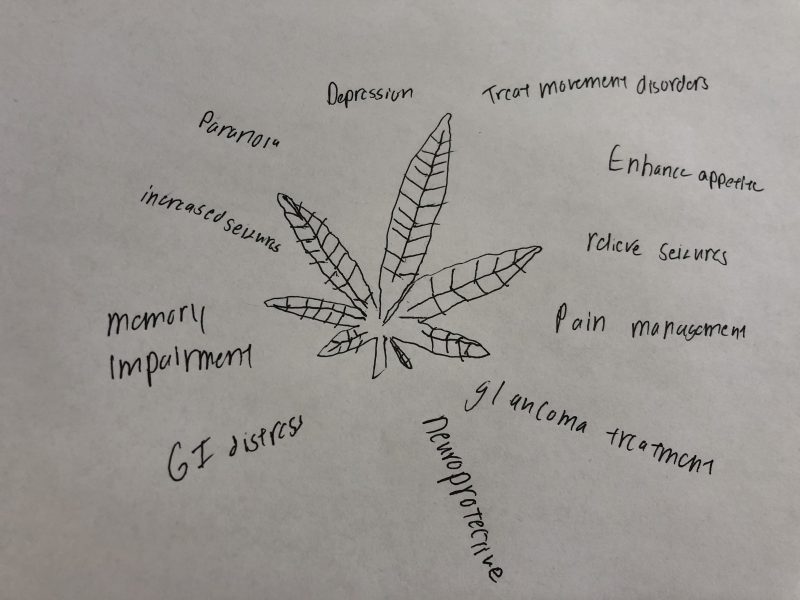
Thee has also been some research suggesting that cannabis derived products can block the pain pathway and might be a solution for people suffering from chronic pain. Some research has also looked a the treatment of movement disorders, including those seen as a result of Parkinson’s, Multiple Sclerosis, and tardive dyskensia and found that the cannabis derived products can help reverse some of the uncontrollable movements that are seen in these disorders.
https://www.ncbi.nlm.nih.gov/pubmed/29533978
However, as the feature image on this post suggests there is concern about the potential for side effects as a result of the use of cannabis-derived products. These concerns include a potential for an increase in the number of seizures, memory impairments, paranoia, and depression. However, because cannabis is listed as a schedule 1 drug by the DEA this limits the amount of research that can be conducted. Schedule 1 implies that there is no medical use for the drug and implies that there is no use in studying potential medical uses. This limits the amount of studies that can be done on the use of cannabis-derived products and our understanding of their effects.