What is Glioblastoma?
In short, glioblastoma (GBM) is brain cancer. More specifically, it is a very lethal brain tumor, sometimes becoming invasive to other parts of the body, such as the spine. There are two different forms that GBM can take: primary and secondary. In their article, Understanding and exploiting cell signaling convergence nodes and pathway crosstalk in malignant brain cancer, Fang and associates define these two types of brain tumor. Primary tumors develop quite fast, most of the time without any symptoms that show the development of the tumor. Secondary tumors grow from smaller tumors until they become malignant. In Figure 1, it can be seen how primary tumors just “show up” with no warning, and secondary tumors grow and grow.

GBM biology
GBM can be classified into two main groups, primary and secondary, but those groups can also be divided into four subtypes. These four subtypes are classified as Classical GBM, Mesenchymal GBM, Proneural GBM, and Neural GBM. These tumors are classified into these one of these four groups based on their transcription factors. Understanding the tumor type of each patient could lead to therapeutic techniques and precise target treatments. Figure 2, pictured below, details each of the four subtypes of GBM. There are different categories for how each subtype is categorized. For example, prognosis for each, different genomic alterations, as well as what proteins may influence these tumors.

Why is it so hard to fight tumors?
Tumors, and cancer in general, can be hard to fight. One of the main reasons GBM is so resistant to therapy is because of the impenetrable blood brain barrier (BBB). The BBB is the brains filtering mechanism, allowing for certain chemicals to pass to and from the BBB, it protects the brain from the bloodstream environment, and provides nutrients that is required for normal functioning. The BBB is composed of cells that are fit tightly together, allowing for some, but not all substances to pass through. To deliver therapeutic drugs to the brain, they need to pass through the BBB. And, if the BBB cannot be penetrated by these drugs, they are not going to be effective. There are many researchers who are trying to address this problem. Many methods have been developed to try and improve permeability of the BBB. Author Quanguo Ho and associates go into detail about these methods in their article, Towards Improvements for Penetrating the Blood-Brain Barrier- Recent Progress from a Material and Pharmaceutical Perspective.

What is a tumor made of?
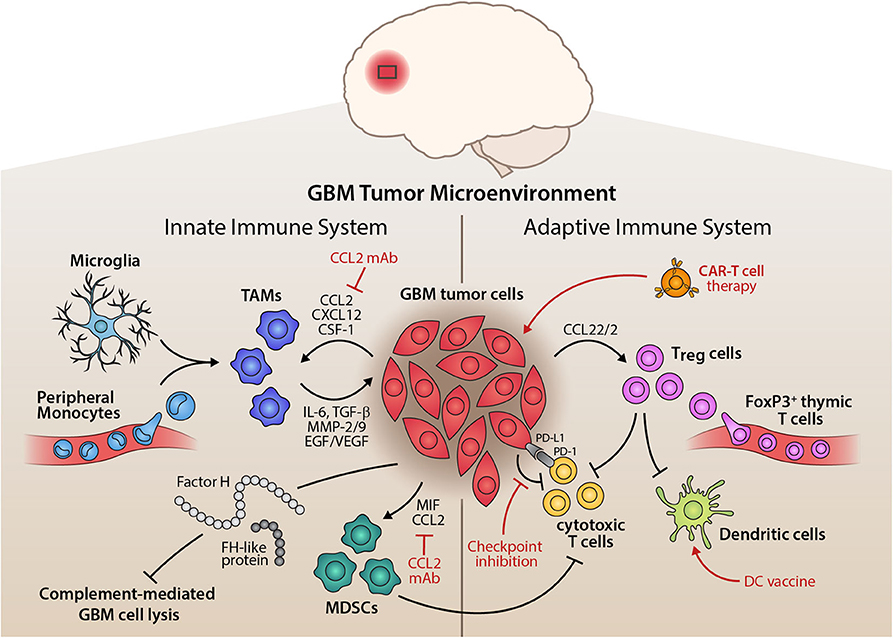
Tumors consist of a microenvironment, the small-scale environment of an organism. In this make up are T-cells, tumor-infiltrating dendritic cells, tumor-associated macrophages, and other complex components. Each environment is heterogenous, or diverse, in its own way. There are no two environments for a tumor that are the same. Each component of these microenvironments acts on each other in different ways. So, tumor survival depends a lot on what is involved in the environment. Figure 4 shows an example of these microenvironments and what is all at play.
Molecular Pathways of a tumor
There are three main molecular pathways that tumors thrive on. All three will be shown below in more detail. These pathways include cAMP, MAPK, and PI3K. Everyone has these pathways in their body, but if something goes wrong, it allows tumors to prosper. For example, if the MAPK pathway becomes phosphorylated, or hyperactive, there is poor patient survival in those with GBM. The PI3K pathway regulates multiple cellular functions within the body. Shown in Figure 6 and Figure 5, there are intricate details to how the pathways operate.
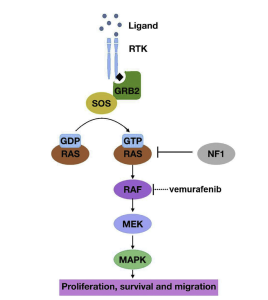
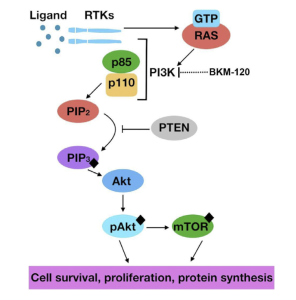
Unlike the MAPK pathway, cAMP and Pi3K are hypoactive. It has been hypothesized that there is most likely a mutation or amplification that occurs on the EGFR protein. This mutation activates other mutations in the pathway which eventually leads to the inactivation of the tumor suppressor gene, PTEN.
The last pathway, pictured in Figure 7, is the cAMP pathway. The two pathways mentioned above regulate multiple cellular functions, cAMP does that as well. However, the cAMP pathway has been studied less than the other two because it seems to be less prominent in tumors. There is a significant reduction in cAMP signaling which may be the reason for tumor production and growth. Figures 5 through 7 show a greater in depth explanation of the three pathways.
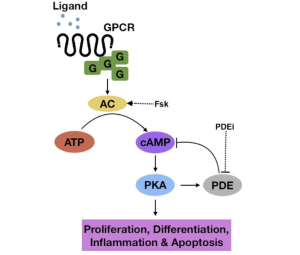
So, what?
With all this information, why should you care? Understanding how these pathways lead to tumor growth is just one way to fight GBM. Specific drugs are able to target different aspects involved in these pathways. But, like mentioned before, the main issue here is the BBB and whether or not the drug can penetrate it. It has been researched that if a specific portion of one pathway is inhibited which results in tumor suppression. However, sometimes the tumor will just migrate and hinder another pathway to help itself grow.
Understanding the mechanism of these pathways and how they and tumors interact with one another will lead to further research in finding a cure for GBM.
References
DeCordova, S., Shastri, A., Tsolaki, A., Yasmin, H., Klein, L., Singh, S., & Kishore, U. (2020, June 01). Molecular heterogeneity and immunosuppressive microenvironment in glioblastoma. Retrieved March 22, 2023, from https://www.frontiersin.org/articles/10.3389/fimmu.2020.01402/full
He, Q., Liu, J., Liang, J., Liu, X., Li, W., Liu, Z., . . . Tuo, D. (2018, March 23). Towards improvements for penetrating the blood-brain barrier-recent progress from a material and pharmaceutical perspective. Retrieved March 22, 2023, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5946101/
Fung NH;Grima CA;Widodo SS;Kaye AH;Whitehead CA;Stylli SS;Mantamadiotis T;. (n.d.). Understanding and exploiting cell signalling convergence nodes and pathway cross-talk in malignant brain cancer. Retrieved March 22, 2023, from https://pubmed.ncbi.nlm.nih.gov/30710631/