Introduction
Glioblastomas (GBMs) are an invasive and aggressive form of malignant brain cancer that are treatment resistant due to the inability of treatments to pass through the blood brain barrier (BBB). GBM formation is either primary or secondary and is classified as four different subtypes. Primary formation occurs de novo, rapidly, and without pre-existing symptoms whereas, secondary GBM develop from low grade glioma tumors. The classification of the four subtypes of GBMs are:
Classical: Amplification of the epidermal growth factor receptor (EGFR) gene and point mutations.
Mesenchymal: Increased rate of neurofibromin 1 (NF1) and phosphatase and tensin homolog (PTEN) mutations.
Proneural: High expression of platelet derived growth factor receptor alpha (PDGFRA) and isocitrate dehydrogenase 1 (IDH1) and TP53 mutations.
Neural: No notable gene amplifications or mutations.
Pathways
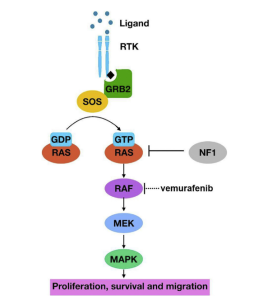
MAPK
Hyperactivation of MAPK through epidermal growth factor (EGF) binding to the respective tyrosine kinase receptor (RTK). The increase activation of the pathway leads to cell proliferation, cell survival, and metastasis resulting in poor patient survival. The hyperactivation of the MAPK pathway results from the inactivation of the NF1 regulator gene.
Figure 1. MAPK pathway
PI3K
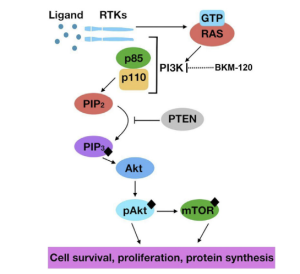
PI3K is made up of two subunits, p110 and p85 and is activated by growth factor stimulation of RTKs. The hyperactivation of the PI3K pathway leads to cell invasion, adhesion, and proliferation. PTEN is used to suppress the PI3K pathway which is mutated.
Figure 2. PI3K pathway
cAMP
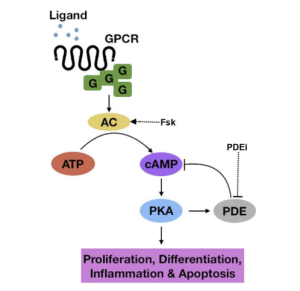
In GBMs cAMP is hypoactivated. cAMP activates PKA which goes and inhibits Raf, ultimately increasing MAPK signaling. The lowered expression of cAMP is a result of altered adenylyl cyclase and PDE expression. Therefore, elevating cAMP via PDE inhibition has been linked to a decrease in tumor growth and promotes GMB cell apoptosis.
Figure 3. cAMP pathway
Pathway crosstalk
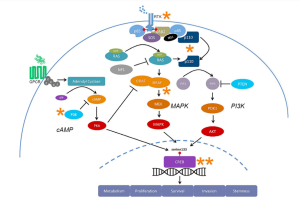
PI3K and MAPK pathways cross regulate each other by both being activated by RTKs and MAPKs proteins Ras and GRB can activate PI3K. Therefore, if only one pathway was targeted to treat the GBM to tumor could manipulate the pathway and continue metastasizing.
Figure 4. Crosstalk between
MAPK, PI3K, and cAMP pathways.
MMPs
Matric metalloproteinases (MMPs) are zinc dependent proteolytic enzymes that are upregulated in the brain in the presences of glioblastomas. Gelatinase MMPs 2 and 9 degrade denatured collagen in result in the degradation of the tight junctions in the BBB, leading to leaking of the BBB. MMP-9 results in tumor growth and both MMP-2/9 are responsible for blood vessel growth. In GBMs blood vessel growth is a negative factor because the tumor redirects blood flow to itself and allows it to continue to grow in size. MMPs are expressed in healthy individuals but are broken down by tissue inhibitors of metalloproteinases (TIMPs).
Treatments
A potential target for treatment would be CREB, since the MAPK, PI3K, and cAMP pathways all converge on CREB. Since CREB is responsible for cell survival, invasion, and proliferation targeting this site may stop tumor cell growth. This target may also lessen the effect of drug resistance since the tumor cannot begin utilizing another pathway to get to CREB. Additionally, CREB is an ideal target because there is less risk for toxicity to occur. However, CREB targeted treatments may still need to be paired with surgery, chemotherapy, or radiation therapy to give the patient the best hope for survival.
Conclusion
Glioblastomas are aggressive and invasive malignant brain tumors that can form as primary of secondary tumors and devastate individuals lives. Since current treatments have been shown to be ineffective at riding the brain of glioblastomas the various pathways involved in GBMs were investigated. The primary pathways are MAPK, PI3K, and cAMP which all converge at CREB. Therefore, targeting CREB may slow tumor growth and spread and give the individual a chance at survival.
References
- Fung, Nok Him; Grima, Corrina A.; Widodo, Samuel S.; Kaye, Andrew H.; Whitehead, Clarissa A.; Stylli, Stanley S.; Mantamadiotis, Theo. Understanding and exploiting cell signaling convergence nodes and pathway cross-talk in malignant brain cancer. Elsevier, 2019.