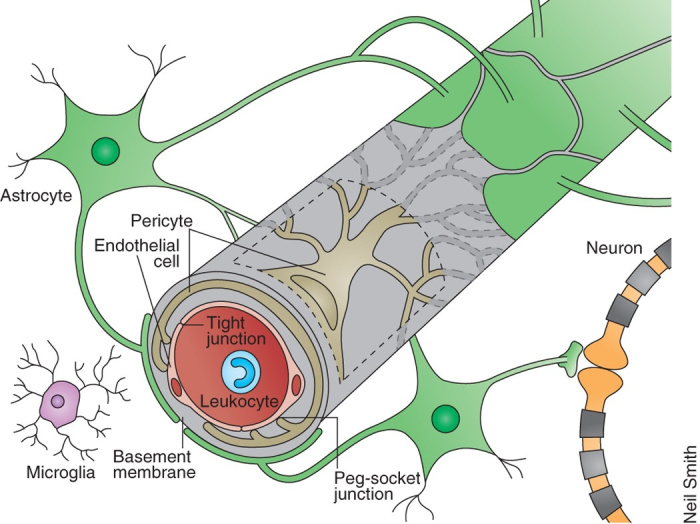
The blood-brain barrier (BBB) is a highly selective and specialized membrane that separates circulating blood from the brain extracellular fluid (BECF) in the central nervous system (CNS). It is composed of tightly-packed endothelial cells lining the capillaries in the brain, as well as surrounding astrocyte cells, pericytes, and basal lamina.
The BBB plays a critical role in maintaining the homeostasis of the brain microenvironment by preventing the entry of many substances from the bloodstream into the brain, including toxins, pathogens, and most drugs.
In glioblastoma, the BBB becomes disrupted and leaky due to the formation of new blood vessels in the tumor, a process known as angiogenesis. The endothelial cells that make up the BBB become damaged and lose their tight junctions, allowing larger molecules to enter the brain. However, the tumor cells themselves can also actively modulate the BBB by secreting various factors that increase its permeability.
The disrupted BBB in glioblastoma can have both positive and negative effects on treatment outcomes. On the one hand, it allows certain therapeutic agents to cross the BBB and reach the tumor, which can improve treatment efficacy. And on the other hand, it allows the tumor cells to evade the immune system and promotes tumor growth in other body parts.
Researchers are actively studying ways to exploit the leaky BBB in glioblastoma to improve treatment outcomes. One approach involves using drugs that can selectively target the tumor cells while minimizing damage to healthy brain tissue. Another approach involves using nanoparticles that can bypass the BBB and deliver therapeutic agents directly to the tumor cells.
Nanocarriers have a wide range of applications. One example is their use in transporting small non coding mRNA molecules that target brain tumor tissue and stop proliferation. The nano carriers protect the RNA from nuclease degradation and promote effective regulation of target genes, especially in brain tumors such as glioblastomas (GBMs) that are somehow protected from chemotherapeutic drugs by the blood–brain barrier (BBB).
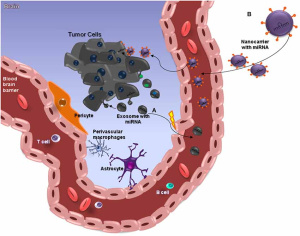
This image attempts to clarify the action of miRNAs in brain-cancer cells, through nano carriers capable of crossing through the BBB.
A question that was brought up in class on Wednesday was whether or not this drug delivery technique is specific to the tumor cells, and does not damage other cells in the immediate vicinity of the tumor, and the answer to that I found in a research study that reviewed the use of CDs, nano particles in drug delivery. The review article reported that the over-expression of transferrin receptors on both tumor cells and the BBB’s endothelial cells allows CDs to be specifically targeted to cancerous cells when conjugated with transferrin.
The authors supported this by referencing Hettiarachchi et al, who designed a triple-conjugated CD-based nano carrier (DDS) that targeted glioblastoma with transferrin, epirubicin, and temozolomide. The results showed that a much lower concentration of the triple-conjugated system (C-dots-transferrin-epirubicin-temozolomide (C-DT)) was required to reduce tumor cell viability compared to non-transferrin systems (NT) and dual-conjugated systems, namely CDs-transferrin-temozolomide (C-TT) and CDs-transferrin-epirubicin (C-ET). These findings suggest that CDs can be loaded with multiple therapeutic agents, resulting in a synergistic effect on antitumor efficiency.
The ideal method for transporting drugs across the BBB would be controllable and not damage the barrier. Among the various presently available approaches, nano-biotechnology-based delivery methods are the most promising .
References:
Dubois et al. Researchgate.net. Retrieved March 24, 2023, from https://www.researchgate.net/publication/273138648_Gliomas_and_the_vascular_fragility_of_the_blood_brain_barrier
Obermeier, B., Daneman, R. & Ransohoff, R. Development, maintenance and disruption of the blood-brain barrier. Nat Med 19, 1584–1596 (2013). https://doi.org/10.1038/nm.3407
Qian, Z. M., Li, H., Sun, H., & Ho, K. (2002). Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacological Reviews, 54(4), 561–587. https://doi.org/10.1124/pr.54.4.561
Zhang, W., Sigdel, G., Mintz, K. J., Seven, E. S., Zhou, Y., Wang, C., & Leblanc, R. M. (2021). Carbon dots: A future blood-brain barrier penetrating nanomedicine and drug nanocarrier. International Journal of Nanomedicine, 16, 5003–5016. https://doi.org/10.2147/IJN.S318732