The human brain is a sophisticated network of impulses that continually adapts to its surroundings. Among its various functions, the endocannabinoid system (ECS) regulates mood, memory, and neurological health. But what happens if the system is disrupted? More significantly, how does the trafficking of CB1 receptors within our cells affect neurological disorders? Kendall and Yudowski (2017)[1] investigate the complicated mechanics of CB1 receptor signaling, offer insight on how these receptors travel throughout neurons, and discuss the potential for illness treatment.
Understanding the CB1 Receptor
CB1 receptors are a type of G protein-coupled receptor (GPCR) that is widely distributed throughout the central nervous system. They are triggered by cannabinoids, which include both endogenous substances like anandamide and plant-derived molecules like tetrahydrocannabinol. When activated, CB1 receptors alter neurotransmitter release, which influences mood, cognition, and pain perception. However, these receptors do not remain stationary within cells; instead, they move about in a process known as receptor trafficking (Figure 1)
Figure adapted from Kendall and Yudowski, 2017[1]
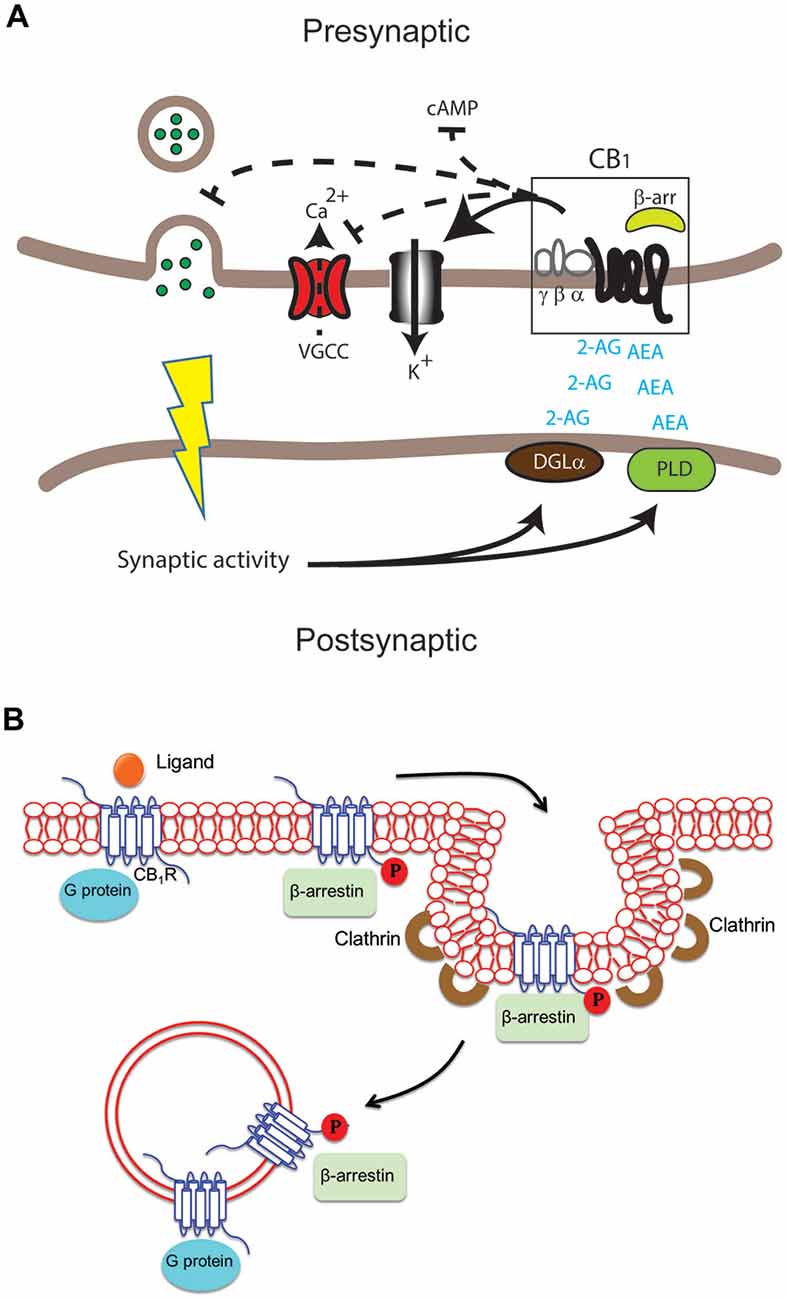
Figure 1. Differential cannabinoid (CB) receptor signaling modalities can impact neuromodulation in health and disease in specific ways. (A)Endogenous ligands arachidonylethanolamine (AEA) and 2-arachidonylglycerol (2-AG) are produced by key enzymes, including diacylglycerol lipase(DGLα) and phospholipase D. These activate the CB1 receptor in the central nervous system (CNS). The outcome may include modification of adenylate cyclase activity to decrease cAMP buildup, voltage-gated calcium channels (VGCC), K+ channels, and neurotransmitter release in presynaptic excitatory and inhibitory synapses. After ligand binding activates the CB1 receptor, signaling through G protein and/or β-arrestin might occur at the plasma membrane, endocytic pits, or endosomes. G proteins typically bind unphosphorylated receptors, whereas β-arrestin binds phosphorylated receptors via G protein receptor kinases[1].
CB1 Receptor Trafficking: A Key to Understanding Disease
When CB1 receptors are activated, they can undergo endocytosis, which is the process by which they move from the cell surface to intracellular compartments. This movement is critical for controlling the strength and duration of cannabinoid signaling. CB1 receptor trafficking is governed by interactions with proteins such β-arrestins, which can either encourage receptor recycling back to the surface or target receptors for destruction [2].
Why does this matter? Research suggests that dysregulation in CB1 receptor trafficking is linked to several neurological disorders, including:
- Alzheimer’s Disease (AD): Impaired CB1 receptor signaling may contribute to memory deficits, as CB1 receptors are involved in synaptic plasticity[3].
- Multiple Sclerosis (MS): Modulating CB1 receptor activity has been explored as a therapeutic approach for reducing neuroinflammation and spasticity[4].
- Huntington’s Disease (HD): A decline in CB1 receptor expression correlates with disease progression, suggesting that preserving receptor function could be neuroprotective[5].
Therapeutic Implications: The Future of CB1 Receptor Research
- The ability to modulate CB1 receptor trafficking presents exciting therapeutic possibilities. By designing drugs that influence receptor movement and signaling bias, researchers aim to create targeted treatments with fewer side effects. For instance, ‘biased ligands’—molecules that preferentially activate either G-protein or β-arrestin pathways—could lead to more selective therapeutic outcomes (Figure 2).
Figure 2. Adapted from Hua et al., “Crystal structure of the human cannabinoid

Figure 2. Synthesis and Characterization of AM6538
(A) Synthetic processes for AM6538[6].
(B) Saturation [3H]-CP55,940 binding assays in the absence (control) or presence of rimonabant (100 nM) or AM6538 (50 nM) show that both antagonists cause displacement of the radioligand’s specific binding when present concurrently in the 1 hr binding assay[6].(C) Membranes were pretreated with buffer (none), rimonabant (100 nM), or AM6538 (50 nM) at 37°C for 6 hours. Membranes were rinsed with buffer 3× before [3H]-CP55,940 binding as described in (B)[6].
(D) The percentage of residual binding (Bmax) was determined using the conditions specified in (B) (concurrent) and (C) (pretreat and wash). When both antagonists were incubated together during the 1 hour binding assay, they reduced [3H]-CP55,940 binding by approximately 30%. Rimonabant has no effect on future radioligand binding under pretreatment or washout conditions, but AM6538 competes even after membrane washing[6].Moreover, understanding the structure of CB1 receptors at the molecular level, as revealed in recent crystallography studies[6], opens doors to designing precise drugs that either enhance or suppress specific receptor functions
Why Should the Public Care?
The public’s interest in the ECS is expanding as cannabis-based medicines become more common. However, without a more sophisticated knowledge of CB1 receptor dynamics, the effects of cannabinoids risk being oversimplified. What’s the takeaway? While cannabinoids show promise, their effects are heavily dependent on receptor signaling and trafficking. Future treatments must account for these complications in order to realize the full potential of cannabinoid-based therapy.
As research progresses, we get closer to understanding the entire therapeutic potential of CB1 receptor regulation. This could open the path for new medicines that benefit millions of people suffering from neurological illnesses.
References:
- Kendall DA, Yudowski GA. “Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease.” Front. Cell. Neurosci. 2017;10:294. DOI:10.3389/fncel.2016.00294
- Delgado-Peraza et al., “Mechanisms of biased β-arrestin-mediated signaling downstream from the cannabinoid 1 receptor.” Molecular Pharmacology. 2016;89(6):618-629. DOI: 10.1124/mol.115.103176.
- Mulder et al., “Molecular reorganization of endocannabinoid signalling in Alzheimer’s disease.” Brain. 2011;134(4):1041-1060. DOI:10.1093/brain/awr046.
- Pryce et al., “Cannabinoids inhibit neurodegeneration in models of multiple sclerosis.” Brain. 2003;126(10):2191-2202. DOI: 10.1093/brain/awg224.
- Mievis et al., “Worsening of Huntington disease phenotype in CB1 receptor knockout mice.” Neurobiology of Disease. 2011;42(3):524-529. DOI: 10.1016/j.nbd.2011.03.006.
- Hua et al., “Crystal structure of the human cannabinoid receptor CB1.” Cell. 2016;167(3):750-762.e14. DOI: 10.1016/j.cell.2016.10.004.