Why Should the Public Care About Antipsychotic Medications?
Antipsychotic medications are a crucial part of psychiatric treatment, helping millions of people manage severe mental health conditions such as schizophrenia and bipolar disorder. Understanding how these drugs work and their impact on brain chemistry is essential for reducing stigma, improving treatment outcomes, and fostering scientific advancements in mental health care. With emerging research linking antipsychotic drugs to complex biochemical pathways like Wnt and GSK3 signaling, the public must stay informed about potential breakthroughs that could lead to more effective and targeted therapies.
Summary of the Science: Wnt and GSK3 Signaling in Schizophrenia
Schizophrenia is a chronic psychiatric disorder that affects brain development and neural connectivity. Despite available treatments, the precise biological mechanisms underlying schizophrenia remain unclear. The article An Emerging Role for Wnt and GSK3 Signaling Pathways in Schizophrenia explores the role of these signaling pathways in the disorder.[1]
Wnt signaling is a crucial pathway in brain development and function. It regulates neural circuit formation, synaptic plasticity, and overall brain health. The research highlights how disruptions in Wnt and glycogen synthase kinase 3 (GSK3) signaling contribute to schizophrenia pathophysiology.
Current antipsychotic drugs, including clozapine and haloperidol, interact with these pathways, affecting dopamine signaling and modulating GSK3 activity. Notably, lithium, a common mood stabilizer, directly inhibits GSK3, potentially stabilizing Wnt signaling and improving psychiatric symptoms.
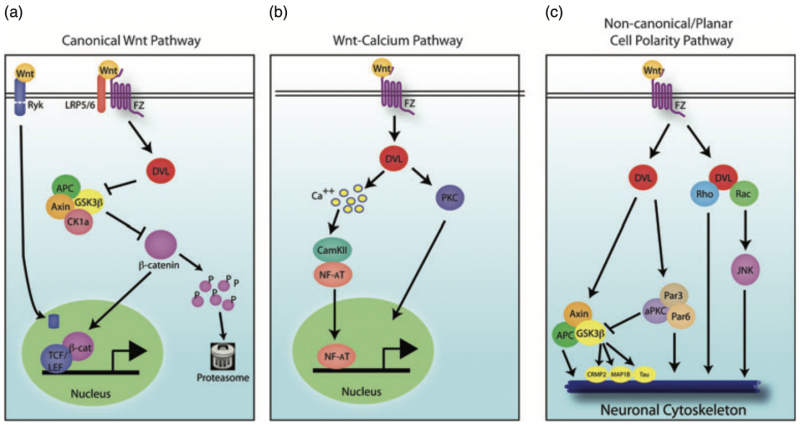
Fig. 1. Wnt signaling pathways. There are three main Wnt signaling pathways: (a) canonical Wnt signaling, (b) Wnt-calcium signaling and (c) non-canonical Wnt/planar cell polarity signaling.
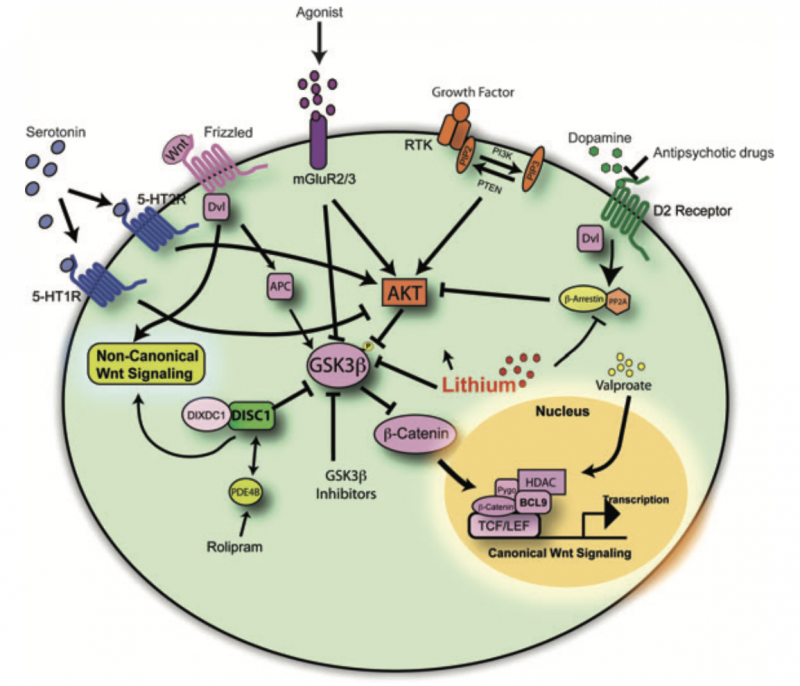
Fig. 2. Psychiatric disease pathways impinge upon glycogen synthase kinase 3 signaling networks. Diagram illustrating how human genetic findings, pharmacological drug treatments directly or indirectly influence canonical and non-canonical Wnt signaling pathways.
Bridging Antipsychotics and Advanced Research
One of the most compelling aspects of this research is the intersection between dopamine and Wnt signaling. Dopamine dysregulation has long been associated with schizophrenia, and emerging findings suggest that Wnt-related mechanisms may further modulate these effects. Studies indicate that D2 dopamine receptor antagonists, a class of antipsychotics, inhibit GSK3, thereby stabilizing β-catenin, a key component in Wnt signaling (Figure 2). This interaction offers a deeper understanding of how psychiatric medications function beyond dopamine receptor blockade.[3]
| Medication | Usage | Benefits | Side Effects |
|---|
| Haloperidol (Haldol) | Used to treat schizophrenia, acute psychosis, and severe agitation. Also used for Tourette syndrome and nausea in some cases. | – Rapid control of acute psychotic symptoms. – Effective in managing severe agitation and hallucinations. – Available in oral, injectable, and long-acting forms. |
– Extrapyramidal symptoms (EPS) such as dystonia, akathisia, and parkinsonism. – Tardive dyskinesia with long-term use. – Increased risk of neuroleptic malignant syndrome (NMS). – Sedation and low blood pressure. |
| Clozapine | Used for treatment-resistant schizophrenia and to reduce suicidal behavior in schizophrenia and schizoaffective disorder. | – Effective for treatment-resistant cases where other antipsychotics fail. – Lower risk of extrapyramidal side effects compared to typical antipsychotics. – Reduces suicidal tendencies in schizophrenia patients. |
– Risk of agranulocytosis (severe drop in white blood cells requiring regular monitoring). – Weight gain and metabolic issues (diabetes, increased cholesterol). – Sedation and excessive drooling. – Risk of seizures at higher doses. |
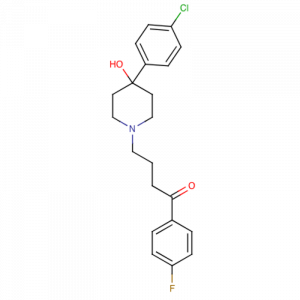
Figure 3: A phenyl-piperidinyl-butyrophenone that is used primarily to treat schizophrenia and other psychoses . It is also used in schizoaffective disorder, delusional disorders, ballism, and tourette syndrome (a drug of choice) and occasionally as adjunctive therapy in intellectual disability and the chorea of huntington’s disease. It is a potent antiemetic and is used in the treatment of intractable hiccups. (From AMA Drug Evaluations Annual, 1994, p279)
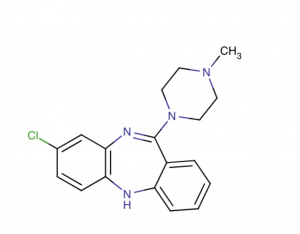
Figure 4: A tricyclic dibenzodiazepine, classified as an atypical antipsychotic agent. It binds several types of central nervous system receptors, and displays a unique pharmacological profile. Clozapine is a serotonin antagonist, with strong binding to 5-HT 2A/2C receptor subtype. It also displays strong affinity to several dopaminergic receptors, but shows only weak antagonism at the dopamine D2 receptor, a receptor commonly thought to modulate neuroleptic activity. Agranulocytosis is a major adverse effect associated with administration of this agent.
Genetic Insights and Future Directions
Recent genetic studies have identified several schizophrenia risk genes involved in Wnt signaling. For example, DISC1, a gene linked to brain development, directly interacts with Wnt signaling proteins, influencing neuronal function.[4] Additionally, AKT1, another schizophrenia risk gene, modulates GSK3 activity, further tying together genetic predisposition and molecular pathways in the disorder.[5]
Understanding these connections could pave the way for new treatment options that go beyond traditional dopamine-targeting antipsychotics. Novel drugs designed to fine-tune Wnt signaling could offer more precise and effective treatments with fewer side effects.[6]
Why This Matters
The public should be aware of these developments because they impact how we approach mental health treatment. By staying informed, we can support scientific research, advocate for improved therapies, and reduce the stigma surrounding psychiatric disorders. Additionally, recognizing that mental illnesses have a biological basis reinforces the importance of medical treatment and continued innovation in drug development.
As research into Wnt and GSK3 signaling progresses, we may witness the advent of next-generation antipsychotics that are more effective, personalized, and capable of addressing the underlying causes of psychiatric disorders rather than just their symptoms. This is an exciting time in neuroscience, and the more we understand, the better we can support those affected by schizophrenia and related conditions.
References:
- Singh KK. “An emerging role for Wnt and GSK3 signaling pathways in schizophrenia.” Clinical Genetics, 2013. DOI: 10.1111/cge.12111
- Clevers H, Nusse R. “Wnt/β-catenin signaling and disease.” Cell, 2012. DOI: 10.1016/j.cell.2012.05.012
- Beaulieu JM, Gainetdinov RR, Caron MG. “Akt/GSK3 signaling in the action of psychotropic drugs.” Annual Review of Pharmacology and Toxicology, 2009. DOI: 10.1146/annurev.pharmtox.011009.081849
- Mao Y, Ge X, Frank CL et al. “Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3β/β-catenin signaling.” Cell, 2009. DOI: 10.1016/j.cell.2009.03.033
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. “Convergent evidence for impaired AKT1-GSK3β signaling in schizophrenia.” Nature Genetics, 2004. DOI: 10.1038/ng1296
- Ripke S, Sanders AR, Kendler KS et al. “Genome-wide association study identifies five new schizophrenia loci.” Nature Genetics, 2011. DOI: 10.1038/ng.940
