Most of us know that a high-fat, high-sugar diet can lead to weight gain. Many people are aware of how obesity is linked to type 2 diabetes, heart disease, and other chronic conditions. But here’s something less commonly known: the food we eat can trigger inflammation in the brain within just a few days, before we even see a change on the scale.
That’s the core insight from the article “Hypothalamic inflammation in obesity and metabolic disease”. This research shows that the brain, particularly the hypothalamus, isn’t just passively responding to weight gain. Instead, it plays an active role in the development of obesity and related diseases.
The Hypothalamus: A Key Player in Energy Balance
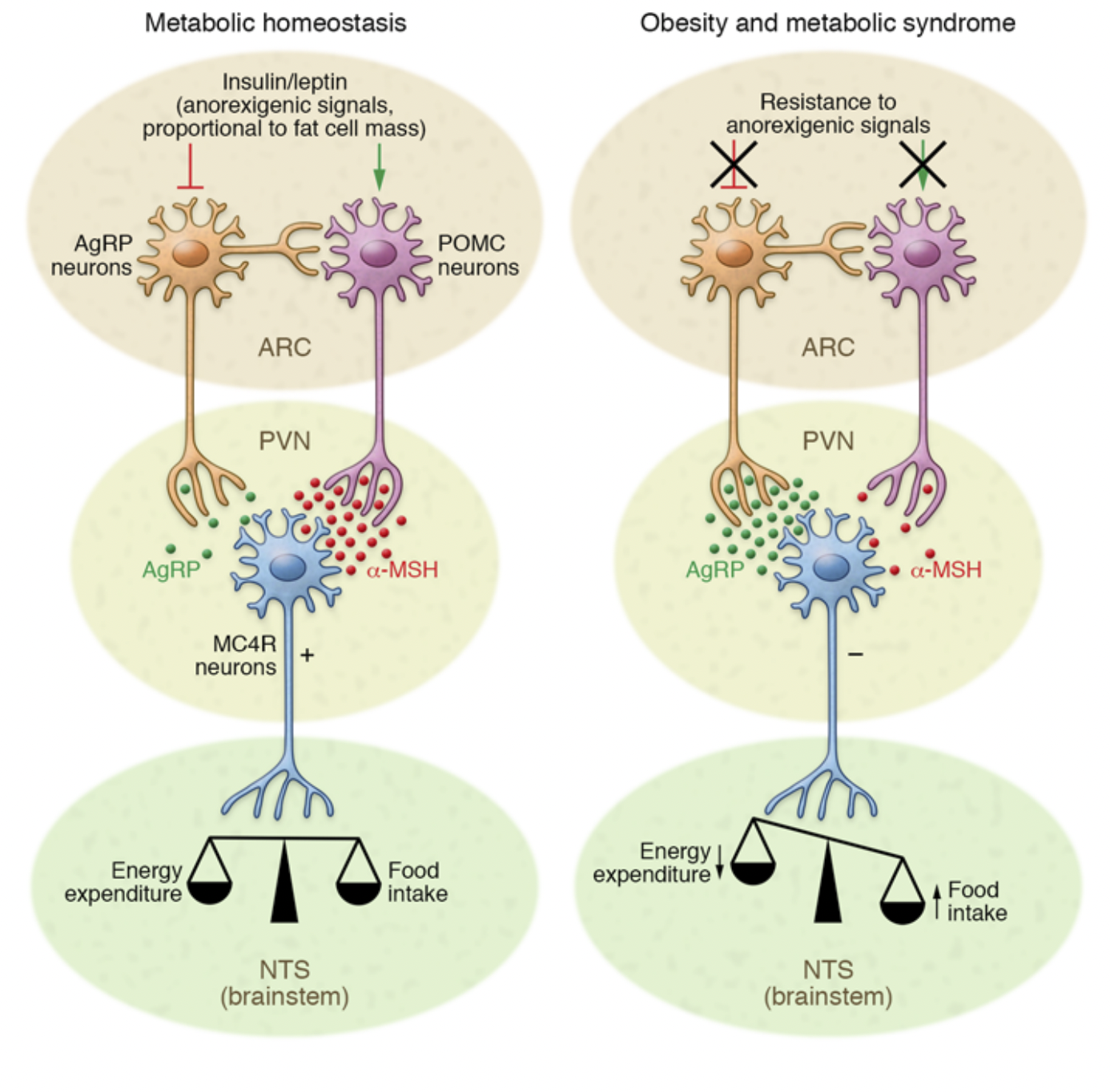
The hypothalamus is a critical region of the brain responsible for regulating hunger, satiety, and overall energy expenditure[1]. As shown in Figure 1, it contains two key types of neurons: POMC neurons, which help reduce appetite, and AgRP neurons, which stimulate it. Under normal conditions, these neurons respond to signals from hormones like insulin and leptin to maintain balance between energy intake and use[1].
However, diets high in saturated fats—particularly palmitic and stearic acids—can disrupt this system quickly. These fatty acids can cross the blood-brain barrier and accumulate in the hypothalamus. Once there, they activate inflammatory pathways like TLR4 and NF-kB, triggering cellular stress and impairing the brain’s ability to respond to leptin and insulin[1]. Essentially, the brain stops receiving the “I’m full” signal, which promotes overeating and weight gain, even before visible changes in body weight occur[1].
Type 2 Diabetes
Type 2 diabetes occurs when the body doesn’t use insulin properly, causing blood sugar levels to rise [2]. This is due to the pancreas producing too little insulin and the body’s cells not responding well to it. Over time, high blood sugar can damage the eyes, kidneys, nerves, and heart[2].
Although once known as “adult-onset diabetes,” type 2 can now affect children, largely due to rising obesity rates[2]. While there’s no cure, the condition can often be managed with weight loss, healthy eating, exercise, and, when needed, medications or insulin therapy[2].
Common symptoms may develop gradually and include:
-
Increased thirst and urination
-
Excessive hunger
-
Weight loss
-
Fatigue
-
Blurred vision
-
Slow-healing sores
-
Frequent infections
-
Tingling or numbness in hands/feet
-
Darkened skin patches, especially in the neck or armpits
Leptin, Obesity, and Type 2 Diabetes
Leptin, a hormone produced by fat cells, plays a central role in appetite control, energy balance, and glucose regulation[3]. In a healthy system, leptin levels rise as fat stores increase, signaling the brain to reduce food intake and increase energy expenditure[3].
But in obesity, something goes wrong: leptin levels are high, but the brain becomes resistant to its effects. This leptin resistance means the brain doesn’t register fullness, which contributes to increased food intake and, over time, insulin resistance a key feature of type 2 diabetes (T2DM). Leptin acts through several neuronal pathways, including GABAergic neurons and cells in the ventral premammillary nucleus of the hypothalamus, though the exact locations and mechanisms are still being studied [3].
Why This Matters
This research challenges the idea that obesity is simply the result of overeating or a lack of willpower. Instead, it highlights a deeper issue: neuroinflammation. The food we eat doesn’t just affect our waistline—it changes the way our brain functions. Inflammation in the hypothalamus can alter how we process hunger and fullness, pushing us toward patterns of overeating and disrupted metabolism.
Understanding this connection opens the door to new therapeutic strategies. If we can find ways to reduce brain inflammation or restore leptin sensitivity, we might be able to intervene early in the development of obesity and metabolic disease, not just treat the symptoms after the fact.
[1]Jais A, Brüning JC. Hypothalamic inflammation in obesity and metabolic disease. J Clin Invest. 2017 Jan 3;127(1):24-32. doi: 10.1172/JCI88878. Epub 2017 Jan 3. PMID: 28045396; PMCID: PMC5199695.
[2] Mayo Clinic. (2025, February 27). Type 2 diabetes. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/type-2-diabetes/symptoms-causes/syc-20351193
[3]Manglani, K., Anika, N. N., Patel, D., Jhaveri, S., Avanthika, C., Sudan, S., Alimohamed, Z., & Tiwari, K. (2024). Correlation of Leptin in Patients With Type 2 Diabetes Mellitus. Cureus, 16(4), e57667. https://doi.org/10.7759/cureus.57667
