INSIDE THE TUMOR: THE COMPLEX BIOLOGY OF GLIOBLASTOMA
Glioblastoma (GBM) is a highly aggressive brain cancer that stems from glial cells, these cells known for their supportive role in the brain. This cancer is notorious for its high mortality rate and poor prognosis, and it is often regarded as the deadliest type of cancer. GBM is one of the most challenging cancers to treat, largely due to its resistance to current therapies.
Cell signaling regulates cell behavior; therefore, cell behavior influences tumor development. GBM rewires neuronal networks and intervenes with three common signaling pathways: PI3K, MAPK, and cAMP. The hyper-activation of these pathways drives tumor malignancy. This raises the question: Why is GBM so aggressive compared to other types of cancer?
WHAT IS GLIOBLASTOMA?
In a healthy brain, glial cells regulate their gene expression very precisely to support neurons and maintain homeostasis. However, in glioblastoma, these cells undergo malignant transformation, which is frequently accompanied by the over-expression of certain genes and proteins, such as EGFR (Epidermal Growth Factor Receptor). EGFR is involved in cell signaling pathways that control cell division and survival. When mutations occur, an excess of EGFR appears on cancer cells, causing them to divide more rapidly.
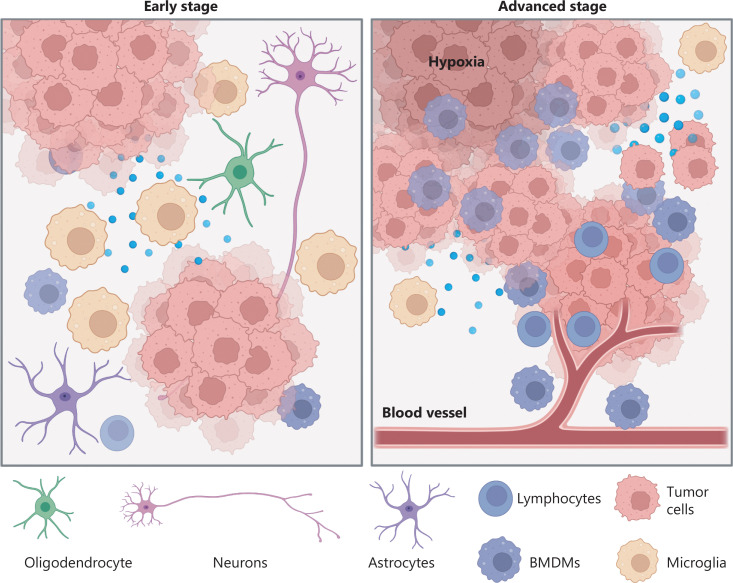
The over-expression of EGFR drives the over-activation of signaling pathways such as PI3K/AKT and MAPK as shown in Figure 1. The tumor environment also has an increasing number of peripheral cells that accumulate within the tumor tissue, further promoting tumor progression. This progression can impair the blood-brain barrier (BBB), as shown in Figure 2. which is the semi-permeable membrane that regulates movement between the blood and the brain. Such impairment can manifest as inflammation.[1]

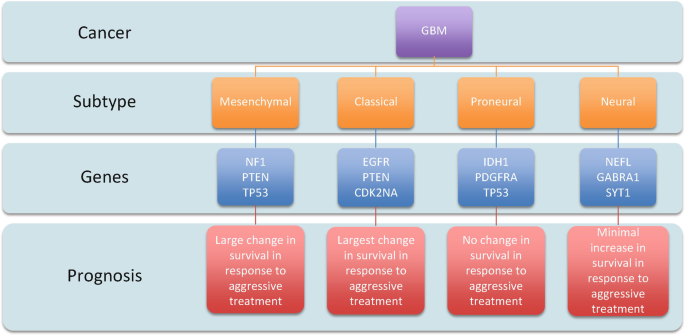
FOUR SUBTYPES OF GLIOBLASTOMA
Classical GBM:
- Amplified EGFR signaling but minimal TP53 mutations.
- Responds well to aggressive treatment.
Mesenchymal GBM:
- Frequent NF1 and PTEN mutations, along with altered MAPK and PI3K pathways.
- Aggressive therapy improves survival.
- longer average survival time
Pro-neural GBM:
- Common in younger patients, with mutations like IDH1 and TP53 and amplified PDGFRA.
- Longer survival but poor response to aggressive treatments.
Neural GBM:
- Characterized by neuronal gene expression, with no obvious mutations.
- Offers the worst survival prognosis.
- symptoms only slightly improve with treatment
HOW GLIOBLASTOMA DISRUPTS NEURONAL SIGNALING
Glioblastoma invades the brain, rewiring neuronal networks and disrupting signaling:
PI3K and MAPK Pathways
- These pathways use cellular signaling to regulate cell proliferation and survival. In GBM, PI3K and MAPK are cross-regulated, which poses a challenge for research because it becomes difficult to target a singular pathway.
cAMP
- cAMP is distinct from the PI3K and MAPK pathways because it generally exerts tumor-suppressive effects rather than driving cell proliferation. cAMP, which stands for cyclic Adenosine Monophosphate, is produced by adenylyl cyclase in response to various extracellular signals. Elevated levels of cAMP activate protein kinase A (PKA), which then phosphorylates downstream targets that promote cell cycle arrest, differentiation, and apoptosis. In contrast, the PI3K and MAPK pathways typically generate proliferative signals that support tumor growth.
WHAT IS CROSSTALK
Crosstalk is a term that indicates the interaction between different signaling pathways. In GBM, a tumor will disrupt the communication between the pathways. dysregulated pathways—such as PI3K/AKT, MAPK, and cAMP—don’t operate in isolation. Instead, they interfere with and amplify each other’s signals, leading to a network of communication that is difficult to shut down with targeted therapies. [2]
TREATMENT CHALLENGES
Despite decades of intense research, GBM remains one of the most challenging cancers to treat. Drug resistence is driven by mulitple factors in GBM, making it difficult to achieve lasting therapeutic responses. As targeting GBM is a major focus of research, two main strategies have been pursued:
EGFR
Researchers have investigated EGFR inhibitors to counter the over-expression of EGFR, which promotes rapid cell division and survival. However, clinical results with EGFR inhibitors have often shown limited benefits, partly because the drugs are unable to fully inhibit downstream signaling and overcome intrinsic resistance mechanisms.
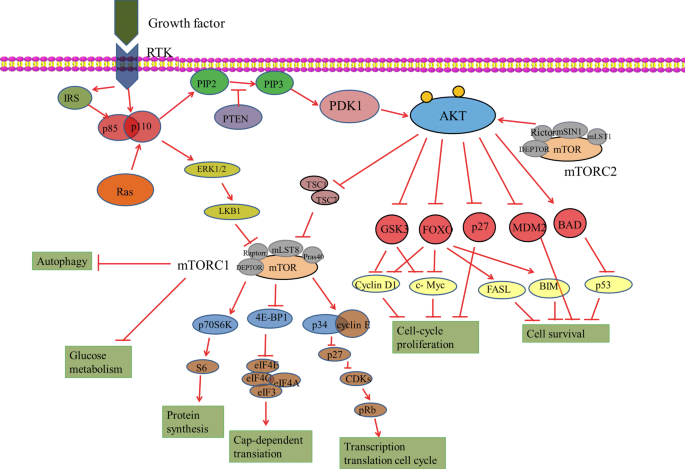
PI3K/AKT/mTOR
Regulatory PI3k/Akt/ mTOR as shown in Figure 4. This pathway supports cell proliferation, survival, and metabolic reprogramming such as promoting glucose dependency. Glioblastoma cells leverage PI3K/AKT signaling to enhance glucose metabolism, which facilitates rapid growth while protecting them from apoptosis. A hyper-activation of PI3K, often combined with the loss of PTEN function, drives this process. The frequent occurrence of PI3K hyper-activation, PTEN loss, and AKT mutations underscores the central role of this pathway as a key driver of oncogenesis and treatment resistance in GBM.[3]
WHY YOU SHOULD CARE!
Understanding the complex signaling pathways involved in GBM is crucial because it explains the underlying reasons for its aggressiveness and resistance to therapy. With GBM being one of the deadliest cancers and given its ability to rewire neuronal networks and activate multiple survival pathways simultaneously, finding effective treatments remains one of the greatest challenges in oncology. Overcoming these challenges could lead to significant advancements in therapeutic strategies and ultimately improve the survival rate and quality of life for patients with this devastating disease.
REFRENCES
[3] Barzegar Behrooz, A., Talaie, Z., Jusheghani, F., Łos, M. J., Klonisch, T., & Ghavami, S. (2022). Wnt and PI3K/Akt/mTOR Survival Pathways as Therapeutic Targets in Glioblastoma. International journal of molecular sciences, 23(3), 1353. https://doi.org/10.3390/ijms23031353
[2] Fung NH;Grima CA;Widodo SS;Kaye AH;Whitehead CA;Stylli SS;Mantamadiotis T; (n.d.). Understanding and exploiting cell signalling convergence nodes and pathway cross-talk in malignant brain cancer. Cellular signalling. https://pubmed.ncbi.nlm.nih.gov/30710631/
[1] Wu, L., Chai, R., Lin, Z., Wu, R., Yao, D., Jiang, T., & Wang, Q. (2023). Evolution-driven crosstalk between glioblastoma and the tumor microenvironment. Cancer biology & medicine, 20(5), 319–324. https://doi.org/10.20892/j.issn.2095-3941.2022.0771