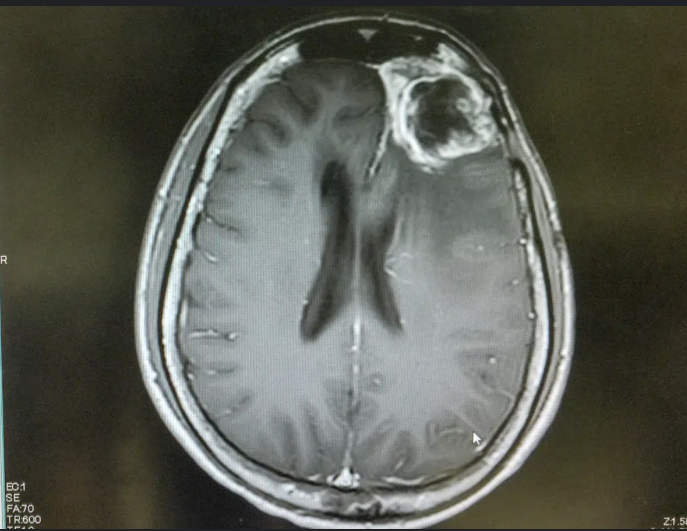
Glioblastoma (GBM) is one of the most feared devastating diagnoses in modern medicine. It’s fast, evasive, and almost universally fatal. But in recent years, researchers have begun to decode its molecular machinery – finally learning to speak the language of this elusive enemy. And that breakthrough could be our best shot yet at out-smarting it. [1]
Why Should We Care?
Chances are, you know someone who has battled cancer. But GBM isn’t just any cancer. It’s uniquely cruel – its survival rate is abysmal, with the average patient living just 14 to 16 months after diagnoses, even with aggressive treatment, Surgery, radiation, and chemotherapy can slow it sown, but rarely stop it. And almost always, it comes back. So why isn’t science doing more? The truth is – it is. And it’s starting to get a whole lot smarter about how.
Thinking Like a Tumor: How GBM Outsmarts Us
A recent study published in Cellular Signaling offers a bold new look at how glioblastoma operates at the molecular level. Rather than examining one pathway at a time, this research explore how GBM hijacks and connects three of the body’s most important signaling routes: PI3K, MAPK, and cAMP. [1]
These pathways normally help healthy cells grow, survive, and communicate. But GBM twists them to its advantage – creating escape routes when one is blocked and adapting rapidly to our best drugs.
The Tumor’s Toolbox: PI3K and MAPK Pathways
Think of these two pathways as power lines delivering survival and growth instruction straight to the cancer cell’s command center.
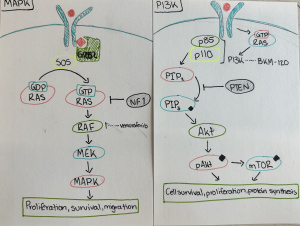
- PI3K Pathway: This one’s like a growth accelerator. It’s often switched on in GBM due to mutations in genes like EGFR (a growth signal receptor) or PTEN (a tumor suppressor that’s often lost). When PI3K is activated, it promotes unchecked growth, survival, and even resistance to treatment. (Figure 1)
- MAPK Pathway: This cascade is all about movement and multiplication. It often starts with the activation of RAS and RAF, leading to a series of events that turn on genes promoting proliferation and migration. One of its downstream targets is the transcription factor CREB3, which we’ll come back to. [2] (Figure 1)
The Underdog Pathway: cAMP
Now here’s where it gets interesting. Unlike PI3K and MAPK, the cAMP pathway often works against cancer. When activated, it can slow down cell division and even trigger apoptosis – programmed cell death. GBM tumors, being clever, tend to suppress this pathway. But researchers are learning how to fight back.
Drugs that boost cAMP – like PDE inhibitors or agents like forskolin – have shown real promise in lab studies. By reactivating this pathway, scientists have managed to push cancer cells toward self-destruction.[4] (Figure 3)
The Bottleneck: Where it All Converges – CREB
Here’s the twist: All three pathways – PI3K, MAPK, and cAMP – feed into one key control center in the cell: CREB (cyclic AMP response element-binding protein). This transcription factor plays a central role in deciding whether a cell grows, adapts, or dies. [5] (Figure 4)
- When CREB is activated by PI3K or MAPK, it ramps up the expression of survival and metabolism genes.
- When activate through cAMP, it can trigger an entirely different set of responses – ones that often suppress tumor growth.
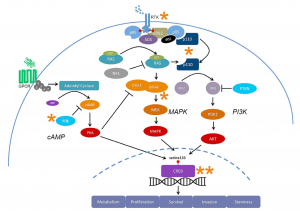
Figure 4 [1]: Key signaling pathways (PI3K, MAPK, and cAMP) in glioblastoma converging on the transcription factor CREB, a critical hub influencing tumor cell survival and apoptosis.
Why This Matters
Cancer is a master of adaptation. Block one path, and it finds another. But what if we could block all the exits? That’s the promise of this new systems-level research: instead of chasing cancer down one road at a time, we start understanding the map. [1]
And we’re already seeing this approach in action. Clinical trials are testing targeted therapies like:
- BKM120 (a PI3K inhibitor)
- Vemurafenib (a BRAF inhibitor targeting the MAPK pathway)
- Rolipram and other PDE inhibitors (boosting cAMP levels)
These drugs are part of a new era of cancer therapy – one that doesn’t just read the tumor, but understands and anticipates its moves.
So Where Do We Go From Here?
There’s still a long road ahead. GBM remains one to the hardest cancers to treat. But this research gives us something we’ve been missing for too long: a strategic roadmap.
The future of glioblastoma therapy may lie in combination approaches – simultaneously targeting multiple pathways, or central hubs like CREB that serve as traffic controllers for tumor behavior. It’s a shift toward systems biology, and it’s exactly the kind of big-picture thinking we need.
As students, researchers, and curious citizens, we should be excited – and supportive – of this work. These breakthroughs don’t just happen in high-tech labs. They happen because people care enough to keep asking the hard questions.
The Bottom Line
GBM has been one of cancer’s greatest enigmas. But we’re getting closer to cracking the code. With smarter science, targeted therapies, and a deeper understanding of how cancer thinks – we just might out-smart it.
And that’s something worth caring about.
References
[1] Fung, N. H. et al. (2019). Understanding and exploiting cell signalling convergence nodes and pathway cross-talk in malignant brain cancer. Cellular Signalling, 57, 2–9. https://doi.org/10.1016/j.cellsig.2019.01.011
[2] Mawrin, C. et al. (2003). Prognostic relevance of MAPK expression in glioblastoma multiforme. International Journal of Oncology, 23(3), 641–648.
[3] Artstract created by Ella Alsleben
[4] Daniel, P. M., Filiz, G., & Mantamadiotis, T. (2016). Sensitivity of GBM cells to cAMP agonist-mediated apoptosis correlates with CD44 expression and agonist resistance with MAPK signaling. Cell Death & Disease, 7(12), e2494.
[5] Mantamadiotis, T. et al. (2012). CREB signalling in neural stem/progenitor cells: recent developments and the implications for brain tumour biology. BioEssays, 34(4), 293–300.