While we have made numerous advancements in the past few decades, there is likely much more we don’t know, let alone understand, about the human brain than what we do. The field of neuroscience was not well established until the 1960s, and though we have made exponential leaps in knowledge of the brain since then, new discoveries often expose more gaps in knowledge. As one neuroscientist, Tom Sudhof, MD, PHD, has put it, “we are still in need of an understanding of the fundamentals” in order to understand elusive phenomena such as consciousness. “There is never a single discovery that changes science… science works as a process that extends over decades.”
A fundamental finding made in 1978 was the presence of insulin receptors in the brain, but it was not until the early 2000s that the role of insulin in the brain became a focus of research. In fact, the brain was classically thought to be insulin-insensitive (not affected by insulin), even after insulin receptors in the CNS were discovered. It’s fairly common knowledge that insulin plays an important role in PNS glucose regulation, but insulin is now understood to be involved in a wide variety of brain mechanisms as well, such as regulation of energy homeostasis in the brain, feeding behaviors, mood, and neuroprotection.
Another prominent role of insulin lies within learning and memory. These two phenomena within the brain are thought to be largely facilitated by a mechanism called synaptic plasticity, and insulin has been shown to interact with this process in a couple of different ways. Some studies have found that insulin can induce Long-Term Depression (LTD), which weakens synaptic strength based on its level of activity, through internalization of a type of glutamate receptors called AMPA receptors. Others show that administration of insulin enhances NMDA receptor glutamatergic transmission (associated with Long-Term Potentiation (LTP), which enhances synaptic strength also based on level of activity. These two components of synaptic plasticity have opposite effects, but they work together to process and store information in the brain. Which aspect insulin modulates may just depend on what subtype of receptor it interacts with.
When insulin in the body doesn’t function correctly, diabetes can result; when insulin signaling in the brain is impaired; several lines of evidence indicate that this may contribute to Alheimer’s disease. Interestingly, the two diseases may be connected, with Type 2 diabetes increasing risk for developing Alzheimer’s disease. As a brief overview, there are many ‘pieces to the puzzle’ for the etiology of Alzheimer’s, but they seem to all center around insulin resistance, which occurs when cells don’t respond properly to insulin. This insulin resistance, then, is hypothesized to be a significant contributor to not only diabetes, but Alzheimer’s as well, serving as a possible link between them.
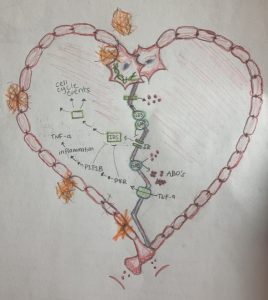
The article Connecting Alzheimer’s disease to diabetes: Underlying mechanisms and potential therapeutic targets (2018) summarizes the many possible contributors to Alzheimer’s development. Three of these aspects are ABOs, gangliosides, and inflammation. ABOs are oligomers of the AB peptide, and function as toxins in the brain when they accumulate. When ABOs phosphorylate IRS-1, part of the insulin pathway needed for proper brain signaling, it becomes inhibited and the signal can’t be properly passed on.
Gangliosides also disrupt insulin signaling, but through different mechanisms than ABO’s. One way is by a type of ganglioside called GM3 disrupting interaction between the insulin receptor (IR) and cav-1, a protein that connects the IR with another substrate necessary for proper insulin signaling. The ganglioside GM1 may also promote insulin resistance by allowing ABO’s to bind to it, resulting in aggregation of these toxins into toxic amyloid structures. Finally, low grade yet chronic inflammation has been seen in brains with Alzheimer’s disease, suggesting that inflammation-mediating cellular pathways and the pro-inflammatory molecules they produce may be involved in Alzheimer development.
We still don’t know exactly how all of these mechanisms fit together, but we are starting to find connections between them that explain how insulin resistance may arise. ABOs activate inflammation signaling, and gangliosides support production and clustering of ABOs, for example. In addition, Inflammation in the form of TNF-a production may perpetuate ABO development. On a broader scale, insulin resistance provides a key link between Alzheimer’s disease, Type 2 diabetes, and metabolic syndrome as well. Understanding this mechanism of pathogenesis does not make these diseases seem any less daunting or complex, but continuing research of and increasing awareness for different factors that contribute to insulin resistance may help society take them more head-on. Perhaps, someday we might be able to prevent them from even occurring at all.