What is Schizophrenia?
Schizophrenia is one of the leading causes of mental disability in the world. On average, schizophrenic patients are 1.0-2.0 standard deviations below the general population’s cognitive ability and they show delay in childhood development milestones. These factors suggest that schizophrenia may likely be a brain development disorder. It’s unclear exactly when the the development is disrupted as current hypothesis range from in utero to late adolescence.
Wnt/B-catenin signaling pathway
Like many cell signaling pathways, the Wnt/B-catenin pathway is very complex and intertwined with many other signaling modalities. For simplicity, I will only discuss the main pathways of Wnt/B-catenin signaling and how it relates to schizophrenia. Although, it should be noted that there are more mechanisms of action for the different enzymes.
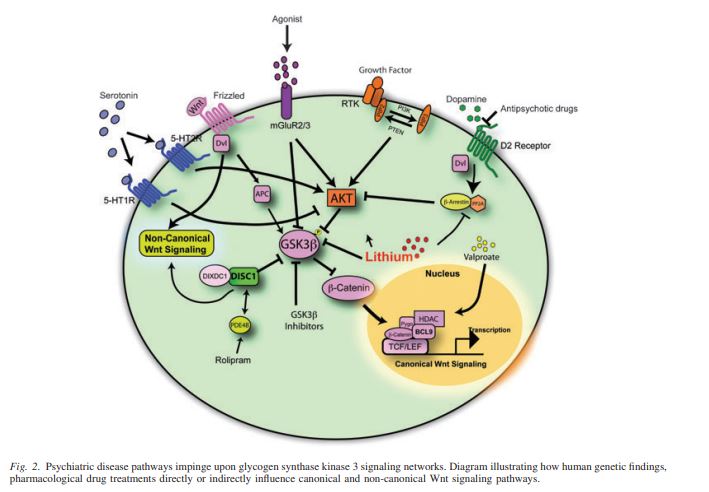
The pathway is first initiated by Wnt binding to its frizzled metabotropic receptor. This causes APC and DVL to migrate towards the cell membrane. As seen in figure 1, APC is part of what we call the ‘B-catenin destruction Complex’. APC and DVL work in tandem to inhibit the destruction complex from degrading B-catenin.
When there is free floating, cytosolic B-catenin, it is able to translocate into the cells nucleus and promote the expression of TCF/LEF. TCF/LEF are transcription factors that lead to genes like Axin, FGF, BMP, and Sox being transcribed. These genes are all important for ensuring proper growth and development of an organism.
Okay, so we have the structural framework of the Wnt/B-catenin signaling pathway, but how does this relate to schizophrenia? To elucidate an answer to this, we must first further complicate our pathway. Our complication comes from the role protein kinase B (AKT) plays in inhibiting the B-catenin destruction complex. AKT expression is regulated by a tyrosine kinase receptor and its growth factor ligand. AKT acts by inhibiting an enzyme within the complex known as GSK3B. When GSK3B is inhibited, the destruction complex is non-functional and cytosolic B-catenin levels begin to increase.
Now, we have enough background knowledge to explain several of the leading molecular hypothesis for the cause of schizophrenia, as well as their treatments. The overarching relationship between schizophrenia and Wnt signaling is that Wnt-signaling is decreased or disrupted. This disruption leads to increased destruction complex activity, decreased B-catenin, and decreased “good” gene expression of TCF/LEF.
One such disruption is too much dopamine (DA) in the extracellular space. This leads to AKT inhibition which, as discussed above, typically inhibits the GSK3B destruction complex. Therefore, Excess DA signaling leads to decreased cytosolic B-catenin able to translocate to the nucleus and promote transcription of TCF/LEF.
Treatments
While there are many treatments for schizophrenia, they are often associated with a host of adverse side effects like tremors, sexual dysfunction, blurry vision, and drowsiness.
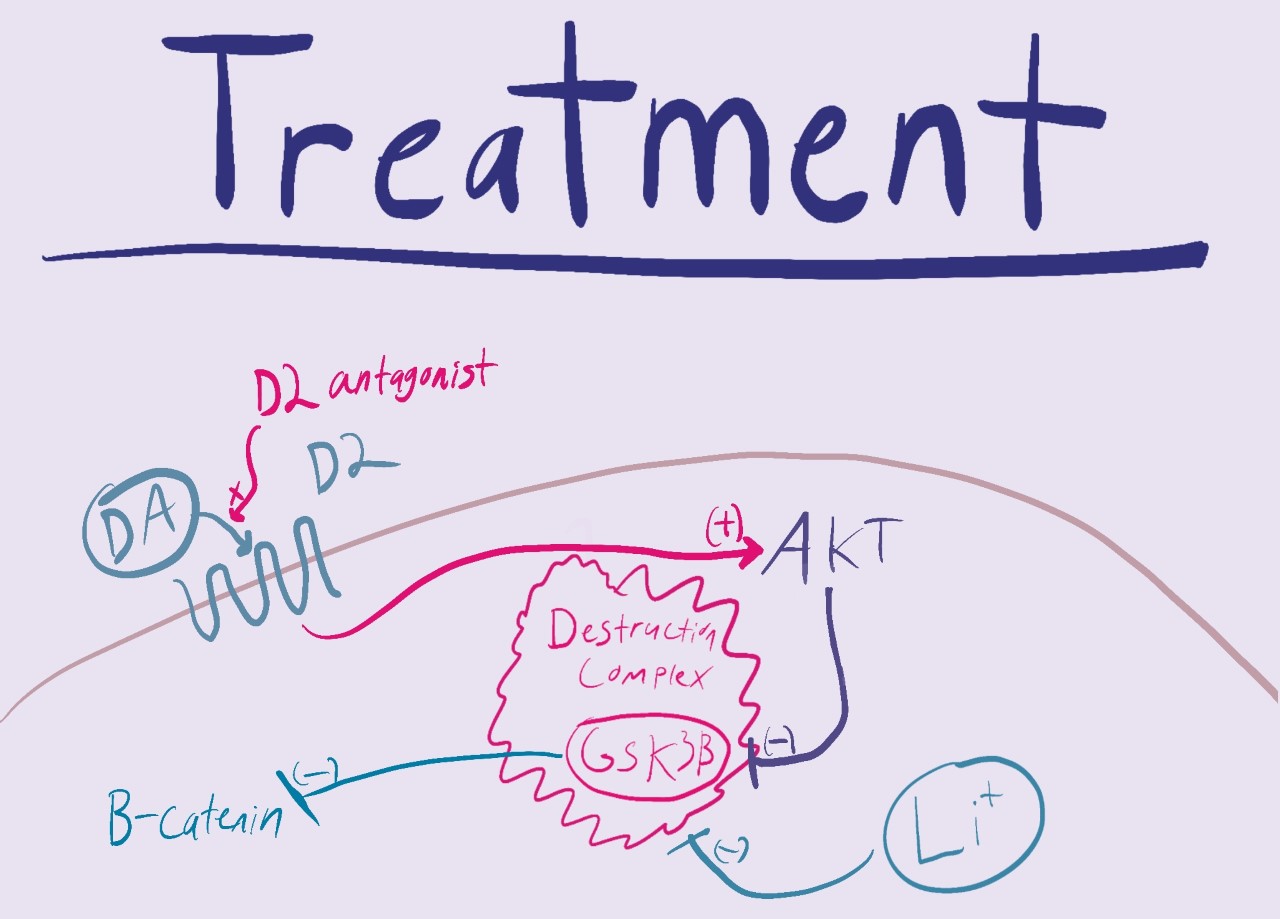
- Li++ inhibits the GSK3B destruction complex by destabilizing it. While much of this mechanism is unknown, it is hypothesized that the Li+ competes with Mg++ for a binding stie on GSK3B.
- Many 1st generation and 2nd generation antipsychotics work as dopamine receptor (D2) antagonists. This means that they prevent DA from binding to its receptor. This Increases intracellular AKT leading to increased B-catenin and TCF/LEF gene transcription.