Different models have been used in order to determine whether an animal is showing signs of schizophrenia due to them being unable to self-report hallucinations, scattered thinking and other features of the disease. These models include developmental, drug-induced, genetic, and sex differences in animal models. Neuropsychiatric disorders, including schizophrenia, include symptoms such as paranoid delusions and auditory hallucinations that are uniquely human and make interpretation of results obtained from animal models more difficult. Increased locomotor activity in response to psychotomimetic compounds such as amphetamine or noncompetitive NMDA glutamate receptor antagonists is commonly used as an indication of positive symptoms in schizophrenia, a deficit in prepulse inhibition (PPI, where a weaker sound prepulse delivered prior to a stronger sound pulse inhibits the natural startle response to the second stimulus) is used as a proxy for sensorimotor gating problems, and changes in social interaction are used as an indicator of negative symptoms.
Developmental Model
The methylazomethanol (MAM) model involves prenatal administration of MAM into pregnant rats. MAM is an antimitotic and antiproliferative agent that methylates DNA47 and acts specifically on proliferation of neuroblasts without affecting glia or producing teratogenic effects in peripheral organs. Administering MAM to pregnant rat’s results in neuroanatomical, electrophysiological, and behavioral changes in the offspring that depend on the gestational day (GD) of administration, with GD17 animals being the most used in schizophrenia animal models.
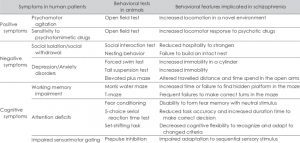
Figure 1. Behavioral tests that are conducted on animals that present positive, negative or cognitive symptoms of schizophrenia and how this effects their results.
Drug-Induced Model
The drugs used most frequently in animal models of schizophrenia are dopamine enhancers (amphetamine and apomorphine) and noncompetitive NMDA receptor antagonists (phencyclidine, ketamine, and MK-801).
Genetic Model
DISC1, a synaptic protein expressed early in development and involved in pre and postnatal development in neurons. DISC1 is considered a highly penetrant mutation and a risk factor for schizophrenia, although the genetic evidence for this claim has been debated. Mice with inducible and/or partial loss of DISC1 function have brain morphology consistent with schizophrenia, including enlarged ventricles and reduced cortical thickness and brain volume.
Sex Differences in Animals Model
Male- or female-specific deficits in PPI in offspring after prenatal PolyI: C have been reported, while deficits in both sexes were found in other studies. Similarly, male-specific deficits have been reported in studies of fear conditioning, set shifting, and recognition memory, while female-specific impairments have been reported in some, but not all, studies of spatial memory in polyI: C offspring.
Conclusion
Although there is no cure to stop schizophrenia, there are current treatments that can control the symptoms. If were are able to understand how different drugs used to alter gene development in pregnant animal models we could figure out where schizophrenia is specifically effecting signaling pathways. This could possibly lead to stoping the gene alterations before birth or see if there are specific drugs that could alter the signaling pathways after birth and stop/weaken the signs and symptoms. Hopefully as research continues to progress more targeted treatments could come along and fix the altered signaling pathways that result in schizophrenia.
Source
https://journals.sagepub.com/doi/full/10.1177/0706743718773728
https://www.researchgate.net/figure/Symptoms-of-schizophrenia-models-and-related-behavioral-tests-in-experimental-animals_tbl2_272403274