TBI and eCBS
Traumatic brain injuries (TBI) are injuries to the nervous system in which many harmful biochemical mechanisms take place. Particularly, there is high intracellular calcium accumulation following the event. Similarly, other harmful cascades may occur including oxidative stress, excitotoxicity, and acute inflammatory responses. It was found that the accumulation of endocannabinoids (eCBs) from the result of injury, including their properties as anti-oxidants, vasodilators, anti-inflammatory agents, inhibitors of excitotoxicity, and their roles as being recognized as self-neuroprotective may be the result of a neuroregenerative response. Both ischemia and traumatic brain injury cause the release of numerous mediators, including harmful factors like glutamate, ROS, pro-inflammatory cytokines, etc., however the eCBS serves as neuroprotectants.
Pathophysiology in TBI and eCBS
A brain injury is a serious event that can wreak havoc on someone’s health, including their perception, emotions, mood, cognition, and sheer ability to function. Following a traumatic brain injury, there are several events of acute pathophysiology, these include, nonspecific depolarization, release of excitatory NT, efflux of K+, increased activity of ATPase, hyperglycolysis to restore ATP, lactate accumulation, Ca2+ influx and sequestration in mitochondria, decreased ATP production, and initiation of apoptosis. All things that are harmful to our health if not controlled and induce inflammation and secondary injury. An excess release of glutamate in the extracellular space following TBI can lead to uncontrolled sodium, potassium, and calcium shifts, which disrupts ionic homeostasis and can lead to cell death. 2-AG, through CB1 activation on nerve terminals, may control neurotransmission, and more specifically, inhibit glutamate release, limiting its excitotoxicity. Given the location of the CB1 receptors on presynaptic terminals of glutamtergic synapses, eCBs and other CB1 agonists could be suggested as a role in neuromodulation following glutamate release, and therefore, as modulators for excitotoxicity in brain disorders.
Also, in the brain, CB2 receptors are expressed mostly in non-neuronal cells, and are up-regulated mainly under neuroinflammatory events. Pro-inflammatory cytokines are released within hours after brain injury. Overall, it has been shown that the eCB system, through acting on each cardiovascular unit, can affect the outcome of TBI through several difference mechanisms. This includes inhibition of inflammatory responses, inhibition of excitatory transmission, and reducing vascular tone. Synthetic cannabinoids can mimic anandamide, 2-AG, and others including CB2 agonists, could be a potential development of drug therapy for the treatment of brain injury.
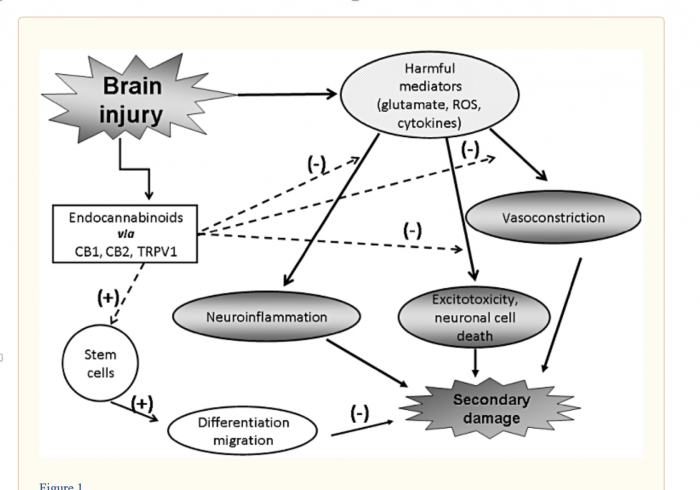
Magic Drug for TBI?
The figure below shows the result of TBI, including neuroinflammation, excitotoxicity, and vasoconstriction, as well as the overall secondary injury that occurs from these responses. After TBI, cells such as neurons, astrocytes, and brain endothelial cells are released which trigger the accumulation of harmful mediators. In the long term, these lead to secondary damage through inflammatory responses and vasoconstriction. Concurrently, there is a “on demand” signal to brain cells to produce eCB, which has been shown to inhibit excitotoxicity, promote antioxidants, and inhibit inflammatory cytokines while countering vasoconstricting effects. Synthetic cannabinoids have been shown to mimic activities of anandaminde, 2-AG, Ara-S, and others, in addition to CB2 agonists, and for this reason, synthetic cannabinoids should be considered as a promising treatment option for traumatic brain injuries.