What is Obesity?
Obesity is a condition characterized by excessive accumulation of body fat to the extent that it impairs health. It is commonly defined by body mass index (BMI), a measure of weight in relation to height, with a BMI of 30 or higher considered obese. Obesity and metabolic diseases such as type 2 diabetes are major public health challenges that have reached epidemic proportions globally. While the exact mechanisms underlying these diseases are complex, recent research has pointed to the role of hypothalamic inflammation in their development.
A paper titled “Hypothalamic inflammation in obesity and metabolic disease” by Alexander Jais and Jens C. Brüning provides an in-depth review of the current understanding of this phenomenon. The authors explain that the hypothalamus, a brain region that plays a crucial role in regulating energy balance and glucose homeostasis, is particularly vulnerable to inflammation due to its high sensitivity to changes in nutrient and hormonal signals.
One section of the paper discusses the mechanisms that contribute to hypothalamic inflammation in obesity and metabolic disease. It also discuss the role of the immune system and how chronic low-grade inflammation can lead to hypothalamic dysfunction.
Fatty Acids
Another interesting aspect of the paper is the discussion of how certain dietary components can influence hypothalamic inflammation. For example, saturated fatty acids, which are commonly found in animal products and many processed foods, can activate inflammatory pathways in the hypothalamus. On the other hand, consumption of omega-3 fatty acids, which are found in foods like fatty fish and flaxseed, has been shown to have anti-inflammatory effects in the hypothalamus.
Neural Networks
Inflammation can affect the neural circuits that regulate feeding behaviour and energy expenditure, leading to alterations in appetite, physical activity, and metabolic rate. Activation of pro-opiomelanocortin (POMC) neurons and inhibition of agouti-related peptide (AgRP) neurons by adipostatic signals leads to the activation of melanocortin 4 receptor (MC4R) expressing neurons in the paraventricular nucleus (PVN) of the hypothalamus. This results in satiety and stimulation of energy expenditure. In contrast, during fasting, AgRP expression increases while POMC expression decreases, leading to decreased MC4R signaling.
Hypothalamic Inflammation
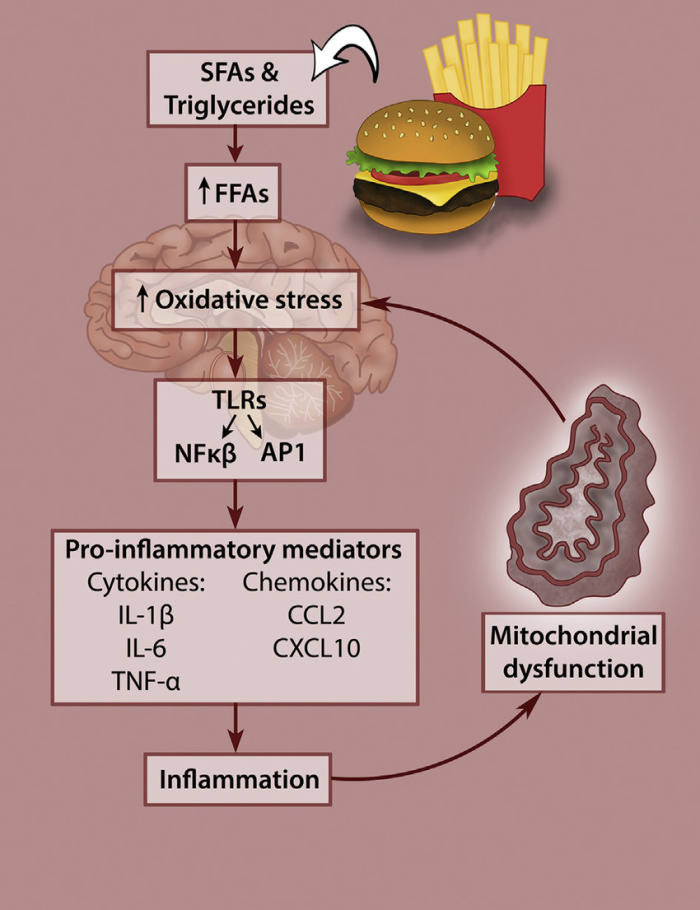
The figure above shows how the consumption of a high fat diet can lead to hypothalamic dysfunction, a condition associated with an increase in oxidative stress. When the hypothalamus becomes inflamed due to the consumption of a high-fat diet, it can lead to a disruption of insulin and Leptin hormones (appetite regulators) and contribute to the development of obesity. Insulin and leptin act directly on specific subsets of neurons in the arcuate nucleus (ARC) of the hypothalamus to control energy balance. The authors describe several approaches, including anti-inflammatory drugs, lifestyle interventions, and dietary modifications, that have shown promise in preclinical and clinical studies.
Overall, this blogpost highlights the importance of understanding the role of hypothalamic inflammation in the development of obesity and metabolic disease. By identifying the underlying mechanisms and consequences of this process, researchers may be able to develop more effective strategies for preventing and treating these conditions.
