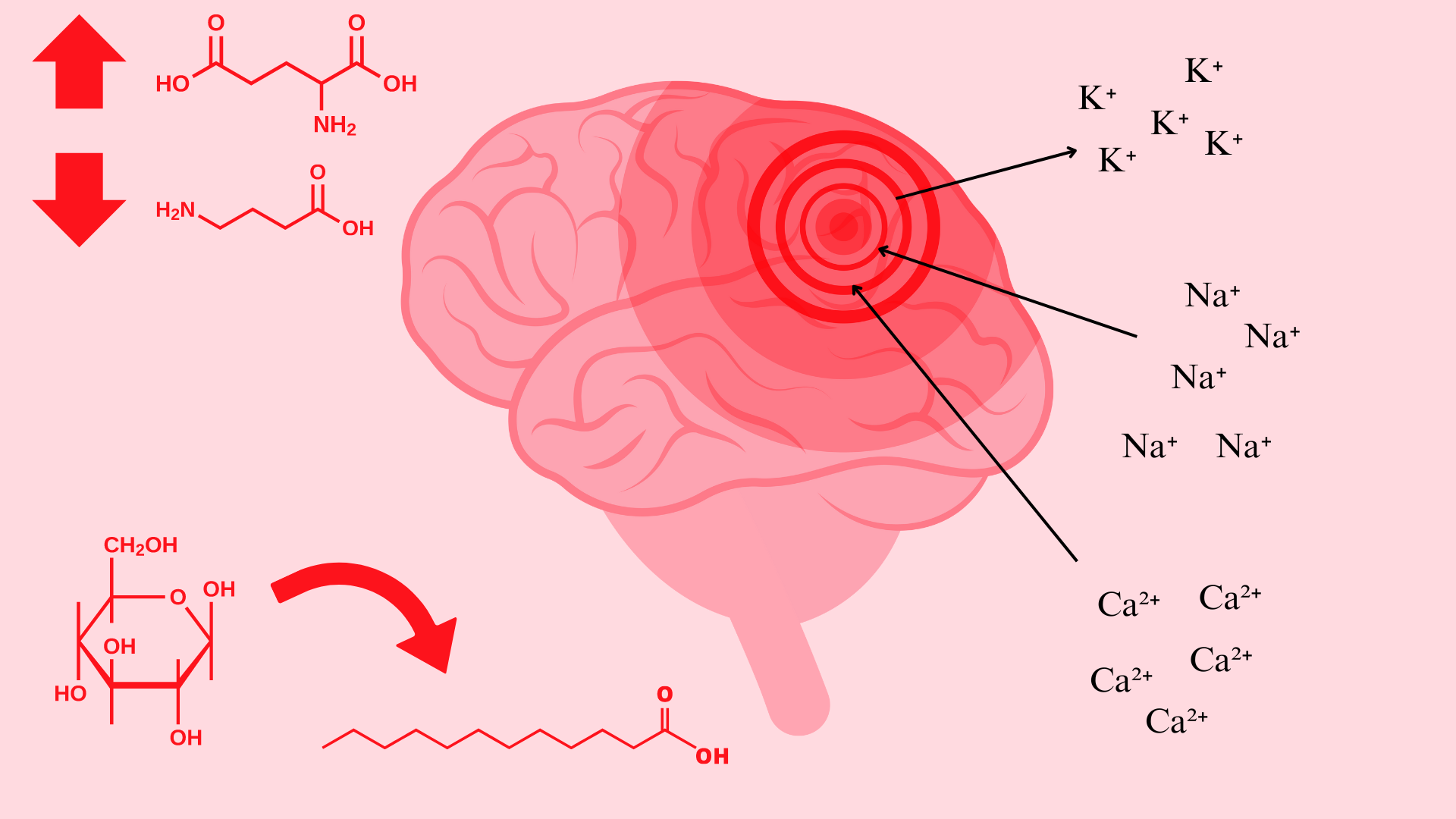
Concussions or traumatic brain injuries (TBI) occur when the head experiences an impact that causes structural damage to brain tissue. Depending on the severity of the injury, this damage may or may not be visible in images of the brain. Despite this, microstructural damage to neural tissue can have significant metabolic and neurochemical effects. This damage can induce chronic cell death and neurodegeneration. Alterations in cellular function can impair neurotransmission, induce metabolic changes, and trigger ionic shifts [1]. Even though these effects can be serious, there is not a universally accepted treatment protocol that modulates these altered pathways.
What Happens After TBI?
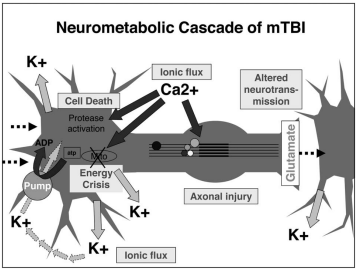
Immediately following a brain injury which results in damage to neural cell membranes, an ionic flux and hyperacute glutamate release can be observed. When the trauma alters the outer membrane of neurons, potassium ions rush out of the cell and sodium and calcium ions rush into the cell, due to the natural ionic gradient between the intracellular and extracellular regions. This initiates a depolarization within the cell, which induces a state of spreading depression which can be a major contributor to acute post-concussive symptoms such as headaches, migraines, and seizures [1].
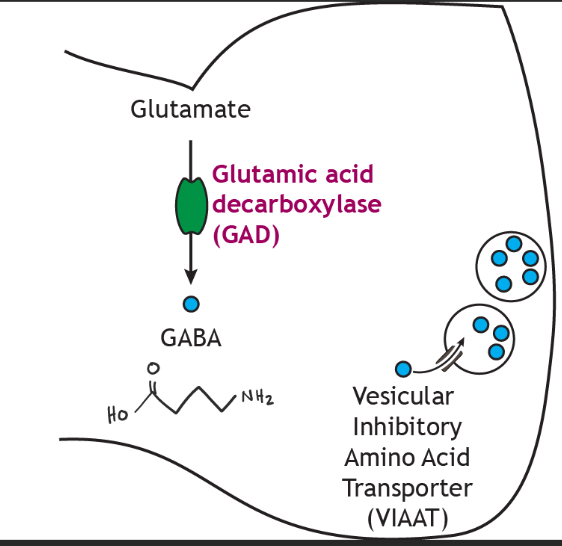
Alterations in GABA, the major inhibitory neurotransmitter, and its receptors have also been observed in TBI models. This could be correlated to the increased susceptibility to anxiety and PTSD following a TBI. GABA transmission is known to produce anti-anxiolytic effects. For this reason, decreased levels of GABA and GAD67 (the enzyme that turns glutamate into GABA) in the amygdala following TBI may specifically contribute to the long-term increases in anxiety observed after brain injury [1].
Likely related to the ionic alterations following structural damage is the metabolic shift that is observed immediately and sustained for 7-10 days following TBI. Immediately following structural damage which induces ionic flux, the cells enter a period of hyperglycolysis in their attempt to return ionic gradients to resting levels. Following this initial increase in glucose metabolism that lasts less than 24 hours, the brain enters a period of impaired glucose metabolism. During this time, it is suggested increased fatty acid consumption could decrease some of the axonal damage that occurs after a concussion [1].
Ketones and Concussions
Fatty acid supplementation could be beneficial following TBI because ketones from the metabolism of fats can be used for fuel instead of glucose in the hypometabolic state. For this reason, it appears that direct ketone supplementation or a ketogenic diet may have more advantageous effects than fatty acid supplementation because ketone body levels are raised more effectively [6].
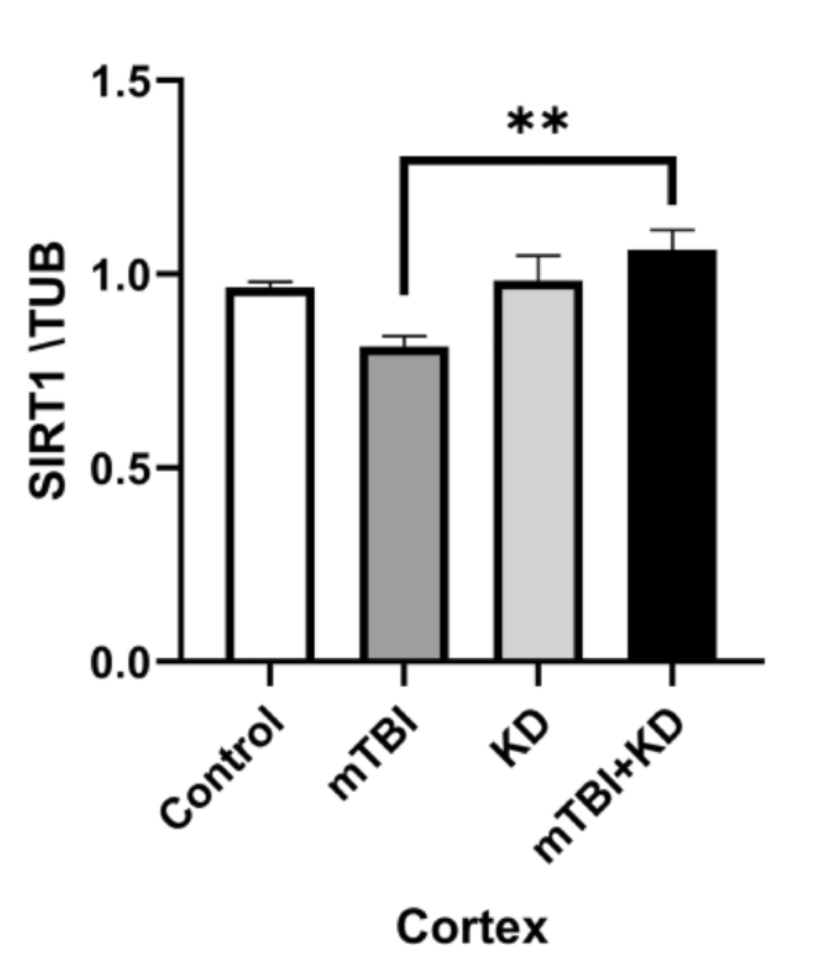
The ketogenic diet (KD) is a high fat, low carbohydrate diet originally used to treat epilepsy. This diet initiates a metabolic state of ketosis where ketone bodies are used for fuel instead of glucose. In a study investigating the potential benefits of a KD following TBI it was shown that compared to a standard diet, a KD produced more ketone bodies, reduced loss of neurons, reduced inflammation, and increased SIRT1 protein following SIRT1 loss due to injury. SIRT1 is a neuroprotective protein that is active in the hippocampus where it activates Akt and inhibits GSK [7].
Given the significant neurochemical and metabolic changes observed following TBI, as well as the vast and serious symptoms this can cause, it is concerning that there are not any highly effective medications or treatments to help heal the structural and metabolic damage that has occurred. The structural damage leads to ionic fluxes which alters brain metabolism, leading to a wide range of behavioral and physiological symptoms. In light of the current understanding of the neural ionic flux and its alterations to neurometabolism, ketone supplementation or a ketogenic diet may be a viable treatment option following a concussion.
References: