Schizophrenia is a complex, debilitating psychiatric disorder that affects millions worldwide. It manifests through hallucinations, delusions, cognitive impairments, and social withdrawal, significantly diminishing quality of life. Despite extensive research, the biological roots of schizophrenia remain elusive, limiting treatment options to managing symptoms rather than addressing underlying causes. Recent advances, however, highlights the importance of Wnt signaling and Glycogen Synthase Kinase 3 (GSK3) pathways, providing new insights that could revolutionize our understanding of schizophrenia and open doors for novel treatments. [1]
Why Should You Care About Wnt and GSK3?
Schizophrenia’s global burden is immense, ranking among the top causes of disability. Treatments currently available – primarily antipsychotics – focus on alleviating positive symptoms like hallucinations but fail to tackle cognitive deficits and the disease’s biological roots. Exploring Wnt and GSK3 pathways not only deepens our understanding of schizophrenia but may also pave the way for targeted therapies that improve long-term outcomes. [1]
Beyond schizophrenia, these signaling pathways are implicated in other conditions like bipolar disorder, autism, and neurodegenerative diseases. Advances in this research may have far-reaching implications for mental health and brain development disorders as a whole. [1]
What’s the Wnt/GSK3 Pathway, and How Does it Connect to Schizophrenia?
Think of Wnt as the brain’s construction supervisor. It helps build the brain during development and keeps neurons communicating properly throughout life. When Wet signaling works, it keeps things in balance by regulating a protein called β-catenin, which helps turn on genes critical for healthy brain function. [1]
Here’s where GSK3 comes in. When Wnt is off, GSK3 tags β-catenin for destruction. But when Wnt is on, it blocks GSK3, saving β-catenin and allowing important genes to do their job (see figure 1).
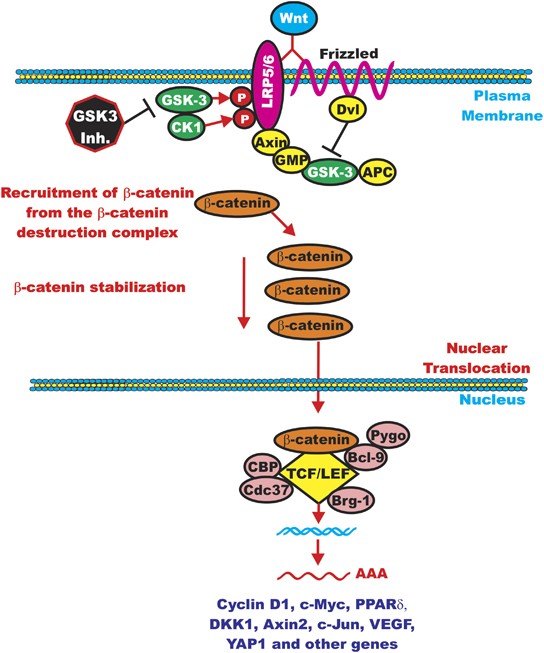
Figure 1: Wnt signaling pathways—showing how GSK3 controls β-catenin levels [2]
In people with schizophrenia, scientists suspect that Wnt signaling is disrupted. That disruption might affect how the brain develops and connects, contributing to symptoms like memory issues and impaired thinking. [1]
Medications Already Target Wnt and GSK3 – Without Us Realizing It
Here’s the kicker: many common psychiatric drugs may already work by influencing this pathway – we just didn’t know it until recently.
For Example (refer to figure 2):
- Antipsychotics like clozapine and haloperidol not only block dopamine (the usual target) but also alter GSK3 activity, changing β-catenin levels. [3]
- Lithium, a go-to treatment for bipolar disorder, directly inhibits GSK3 and boosts Wnt signaling, which might explain its mood-stabilizing effects. [1]
- Newer drugs targeting glutamate receptors (mGlu3/3) also seem to activate Wnt pathways, offering fresh hope for better treatments. [4]
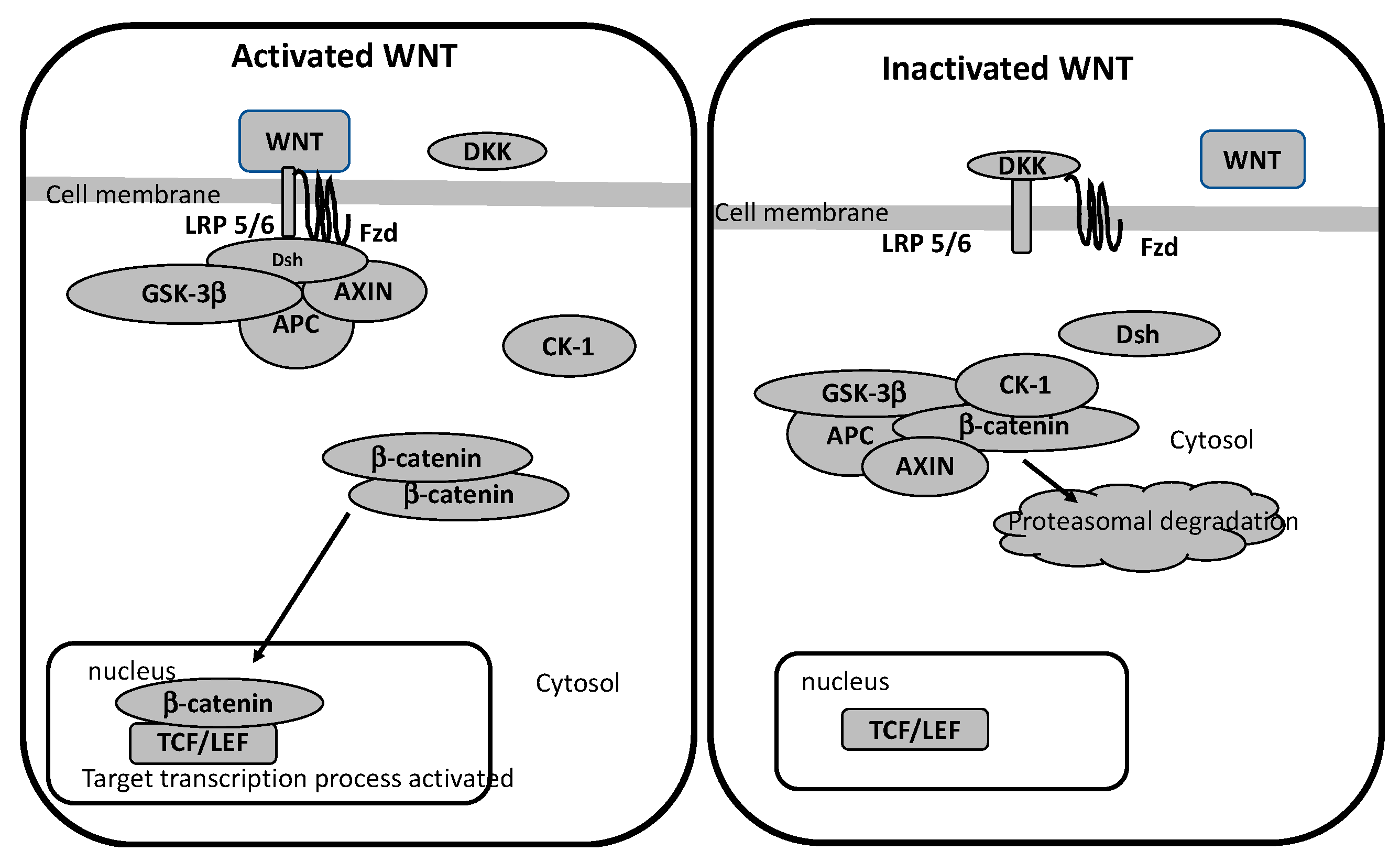
Figure 2: How antipsychotics, lithium, and glutamate drugs influence Wnt/GSK3 pathways [5]
The Genetic Connection: Are Some People Born with Wnt Pathway Risks?
Recent genetic studies show that some schizophrenia risk genes directly affect Wnt signaling:
- DISC1 was first discovered in a Scottish family with a history of schizophrenia. DISC1 interacts with GSK3, regulating β-catenin and impacting brain development.
- AKT1, another gene involved in blocking GSK3, is often found at low levels in schizophrenia patients. [6]
- Researchers have also found mutations and copy number variations (CNVs) in Wnt-related genes like BCL9, which can alter brain size and connectivity.
This genetic evidence strengthens the case that Wnt signaling isn’t just involved – but may be central to how schizophrenia develops. [1]
Animal Studies Back it Up
Mice engineered to have defects in Wnt signaling – like knocking out the Dvl1 gene – show schizophrenia-like behaviors, such as social withdrawal and memory problems. Other mouse models confirm that too much or too little GSK3 activity can trigger hyperactivity, cognitive issues, and mood swings – mirroring what we see inhuman patients. [7]
Where Do We Go From Here?
Research into Wnt and GSK3 is giving scientists a whole new roadmap to understanding schizophrenia. Instead of just treating the symptoms, future therapies might target these pathways to protect the brain, improve cognition, and possibly even prevent the illness from progressing.
And because Wnt and GSK3 are involved in so many brain functions, this research could also unlock treatments for conditions like autism, bipolar disorder, and dementia. The more we understand these pathways, the closer we get to helping those living with these conditions.
References
[1] Singh, K. (2013). An emerging role for Wnt and gsk3 signaling pathways in schizophrenia. Clinical Genetics, 83(6), 511–517. https://doi.org/10.1111/cge.12111
[2] McCubrey, J. A., Steelman, L. S., Bertrand, F. E., Davis, N. M., Abrams, S. L., Montalto, G., D’Assoro, A. B., Libra, M., Nicoletti, F., Maestro, R., Basecke, J., Cocco, L., Cervello, M., & Martelli, A. M. (2013, June 19). Multifaceted roles of GSK-3 and Wnt/β-catenin in hematopoiesis and leukemogenesis: Opportunities for Therapeutic Intervention. Nature News. https://www.nature.com/articles/leu2013184
[3] Freyberg, Z., Ferrando, S. J., & Javitch, J. A. (2010). Roles of the AKT/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. American Journal of Psychiatry, 167(4), 388–396. https://doi.org/10.1176/appi.ajp.2009.08121873
[4] Dogra, S., Stansley, B. J., Xiang, Z., Qian, W., Gogliotti, R. G., Nicoletti, F., Lindsley, C. W., Niswender, C. M., Joffe, M. E., & Conn, P. J. (2021). Activating mglu3 metabotropic glutamate receptors rescues schizophrenia-like cognitive deficits through metaplastic adaptations within the Hippocampus. Biological Psychiatry, 90(6), 385–398. https://doi.org/10.1016/j.biopsych.2021.02.970
[5] Vallée, A., Vallée, J.-N., & Lecarpentier, Y. (2021, April 26). Lithium and atypical antipsychotics: The possible Wnt/? pathway target in glaucoma. MDPI. https://www.mdpi.com/2227-9059/9/5/473
[6] Thiselton, D. L., Maher, B. S., Webb, B. T., Bigdeli, T. B., O’Neill, F. A., Walsh, D., Kendler, K. S., & Riley, B. P. (2009). Association analysis of the pip4k2a gene on chromosome 10p12 and schizophrenia in the Irish study of high density schizophrenia families (ISHDSF) and the Irish case–Control Study of schizophrenia (ICCSS). American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 153B(1), 323–331. https://doi.org/10.1002/ajmg.b.30982
[7] Powell, C. M., & Miyakawa, T. (2006). Schizophrenia-relevant behavioral testing in rodent models: A uniquely human disorder? Biological Psychiatry, 59(12), 1198–1207. https://doi.org/10.1016/j.biopsych.2006.05.008
