Obesity has become a global health concern that is inextricably connected to a variety of chronic diseases, including type 2 diabetes, cardiovascular disease, and neurological disorders. This illness is characterized by low-grade systemic inflammation that affects not only peripheral organs but also the brain, particularly the hypothalamus, which is responsible for controlling energy homeostasis. Within this brain architecture, astrocytes perform an important but understudied function in modulating the effect of high-fat diets (HFDs) on leptin signaling. This review highlights the key findings of Jais and Brüning and explores why these findings should be relevant to the public and scientific sectors alike.¹
Why Should the Public Care?
The public should be aware that the foods we consume can directly influence brain function and overall health. Consumption of saturated fats—abundant in processed and fast foods—not only expands waistlines but also disrupts brain signals that regulate hunger and energy usage. Specifically, this disruption leads to leptin resistance, where the body no longer responds effectively to the “stop eating” signals. Astrocytes, supportive glial cells in the brain, are at the heart of this malfunction. Recognizing this link shifts the blame for obesity from a lack of willpower to a genuine biological alteration—one that begins within days of poor dietary choices.2
Main Goal of the Article
The primary aim of the article by Jais and Brüning is to dissect the role of hypothalamic inflammation in the development of obesity, with an emphasis on how various cell types—including astrocytes—mediate this response to HFDs. The review highlights the temporal sequence of inflammation, demonstrating that hypothalamic changes often precede measurable weight gain and may serve as early drivers of metabolic dysfunction.¹
Astrocytes and Leptin Resistance: A Central Mechanism
Astrocytes, traditionally viewed as supportive cells, are now recognized as active participants in neuroimmune communication. Under HFD conditions, these cells undergo reactive astrogliosis—a transformation characterized by proliferation and inflammatory signaling. Saturated fatty acids (SFAs), especially palmitate, activate toll-like receptor 4 (TLR4) on astrocytes, leading to activation of the nuclear factor-kappa B (NF-κB) pathway. This, in turn, stimulates the release of proinflammatory cytokines such as TNF-α and IL-1β, which inhibit leptin signaling in nearby neurons.³
Leptin, an adipocyte-derived hormone, normally binds to receptors on proopiomelanocortin (POMC) neurons in the arcuate nucleus (ARC) of the hypothalamus to suppress appetite. However, astrocyte-mediated inflammation blunts this response, contributing to a vicious cycle of overeating and weight gain. Importantly, these changes can occur rapidly—within 24 to 72 hours of high-fat intake.⁴
The Hippocampus Is the Region Where Memories Are Stored in the Brain
Did you know that our brains contain highly specialized regions for performing different tasks? For example, there are different areas of the brain for talking, walking, hearing, etc. One of these very specialized regions, known as the hippocampus, is in charge of storing memories and helping us learn [3] Glial cells nourish, protect, and give stability to neurons. In the hippocampus, both neurons and glial cells are critical for storing memories and helping us learn (Figure 1).
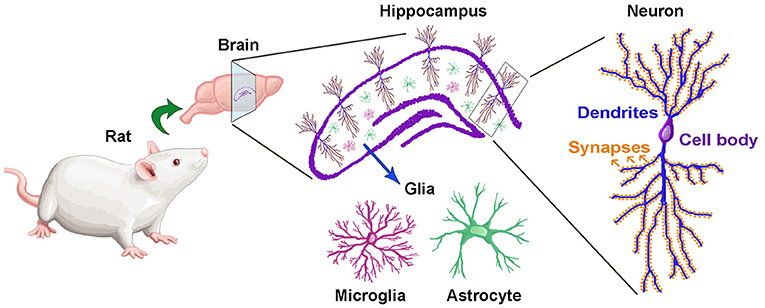
- Figure 1 – The hippocampus is the brain region where memories are made and stored.The location of the hippocampus in the rat brain is shown in purple. If we look at the hippocampus under a microscope, we can see that it is made up of neurons and glial cells. There are two types of glial cells: astrocytes and microglia. Neurons have many specialized regions that allow them to receive and send messages and thus communicate with other neurons. Dendrites are long, branch-like structures that transmit electrical signals in the brain. Synapses are tiny structures at the end of dendrites that help neurons communicate.
Eating a Diet High in Sugar and Fat Has a Negative Effect on Neurons in the Hippocampus
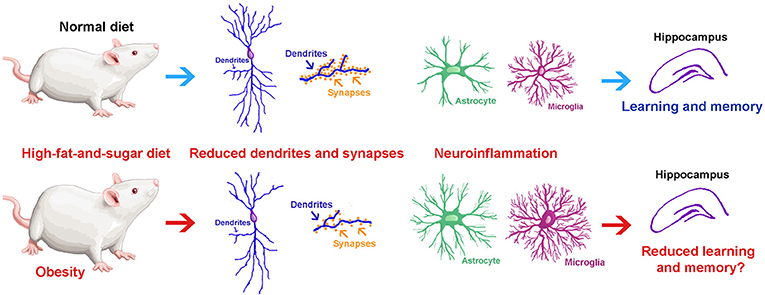
- Figure 2 – in this study they founded out what happens to the hippocampus after rats ate a high-fat-and-sugar diet for 7 days (bottom half) and compared it to rats eating a normal diet (upper half): (1) Neurons have fewer, shorter, and thinner dendrites; (2) Neurons had fewer synapses; and (3) Glial cells became activated by inflammation in the brain. They concluded that eating a diet high in fat and sugar for seven days caused obesity and produced negative effects on the neurons and glial cells of the hippocampus. We believe that these neuronal and glial changes could have a negative effect on memory and learning.
What the Public Should Know
-
Early Impact: Brain changes begin before weight gain is visible.⁴
-
Leptin Resistance: A key hormone in appetite regulation becomes ineffective due to inflammation triggered by dietary fats.
-
Astrocytes Matter: These glial cells are not just “brain glue”; they actively contribute to metabolic disease.
-
Reversible Pathology: Lifestyle interventions, especially dietary changes and exercise, can attenuate these neuroinflammatory pathways.²
Conclusion
The findings reviewed by Jais and Brüning underscore a paradigm shift in obesity research—highlighting the brain, and astrocytes in particular, as key players in diet-induced metabolic dysfunction. Public awareness of how diet influences brain inflammation can drive healthier food choices and inform policies aimed at combating obesity at a structural level. For clinicians and researchers, astrocytes represent a promising target for novel interventions against leptin resistance and metabolic syndrome.
Footnotes
-
Alexander Jais & Jens C. Brüning, Hypothalamic inflammation in obesity and metabolic disease, The Journal of Clinical Investigation, 127(1), 24–32. https://doi.org/10.1172/JCI88878
-
Buckman, L. B., et al. (2015). Evidence for a novel functional role of astrocytes in the acute homeostatic response to high-fat diet intake in mice. Molecular Metabolism, 4(1), 58–63. https://doi.org/10.1016/j.molmet.2014.11.001
-
Gupta, S., et al. (2012). Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. Journal of Neurochemistry, 120(6), 1060–1071. https://doi.org/10.1111/j.1471-4159.2011.07643.x
-
Valdearcos, M., et al. (2014). Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Reports, 9(6), 2124–2138. https://doi.org/10.1016/j.celrep.2014.11.018
-
Calvo-Ochoa E and Arias C (2019) Food for Thought: What Happens to the Brain When We Eat Foods High in Fat and Sugar?. Front. Young Minds. 7:32. doi: 10.3389/frym.2019.00032