Alzheimer’s Disease (AD) is a very debilitating disease that destroys memory and other proper cognitive functions. It can be really hard on families and friends of the patient. It is also tough because there is no known specific cause of AD, although there are some genes that have been found to show some susceptibility to the disease. It is also unfortunate because there is no cure for AD, and most treatments cannot reverse the memory loss and dementia seen with the disorder. The buildup of β-amyloid plaques and neurofibrillary tangles in the brain, along with other protein aggregations, such as tau, are some of the main biomarkers of the disease.
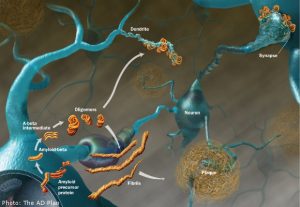
Aging has been identified as a risk factor for AD. The PI3-K(phosphoinositide 3-kinase)/Akt signaling pathway regulates healthy aging and longevity. Its main activators are insulin and IGF-1 (insulin-like growth factor-1). In AD, this pathway is continuously activated, which leads to desensitization and removal of some of the normal insulin/IGF-1 responses in the brain, as well as AD pathology and cognitive decline. Insulin/IGF-1 binds to the receptor tyrosine kinase, which activates PI3-K. PI3-K cleaves PIP2 into PI3. PIP2 binds Atk, which can then go on to phosphorylate things, such as FOXO, GSK3β, and mTOR. The first two are decreased with activation of the pathway, and the latter is increased. Without the normal transcription levels of FOXO, the brain loses some of its defenses against stress and inflammation. mTOR is increased, and it will uncouple the insulin receptor substrate from the insulin receptor, which leads to the insulin resistance seen in AD. It will also increase protein translation at synapses, such as tau and APP (which gets cleaved into Aβ), which may contribute to the proteins aggregations seen in the disease as well. The over-active kinases seen with over-activation of the pathway also contributes to the hyperphosphorylation of tau seen in tau protein aggregations. The over-active PI3-K/Akt pathway leads to Aβ aggregation as well, developing into the β-amyloid plaques and neurofibrillary tangles. These plaques and protein aggregations also contribute to the development of one another and are what are thought to mainly contribute to the pathology of AD as the normal functions of neuronal polarity, synaptic plasticity, learning, memory, metabolic function, and proteostasis are thrown off. They block the normal movement of other molecules important for carrying out these processes from being able to enter the nucleus as well.

Clearance of the β-amyloid plaques seems to be a great solution to curing AD, as that is what is thought to be one of the main culprits of the disease pathology. Currently the main treatment options are acetylcholinesterase inhibitors and NMDA receptor antagonists. There have been some studies to find things that could clear the plaques and the Aβ protein precursor. I found a couple things that have been tried, but mostly these studies have only been done in animal models, namely mice, and not in humans yet.
Possible Treatments for Clearing Aβ:
1. Rosemary extract – rosmarinic acid is an antioxidant, antiviral, antibacterial, and anti-inflammatory. Protects form oxidative stress and is thought to inhibit the formation of Aβ and destabilize and dissolve beta-amyloid fibrils that have already formed. It also has calming effects.
2. Ginkgo biloba extract – an antioxidant, neutralize free radicles, help resist damaging effects of Aβ
3. Red ginseng extract – regenerate brain axons and synapses, reduce inflammation by Aβ and promotes microglial phagocytosis of Aβ

4. EPPS (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) is a zwitterionic organic chemical buffering agent) – known to bind to amyloid-beta aggregations and break them down into single-unit proteins. In mice had better results when taken orally

5. THC – reduced amyloid-beta protein levels and helped eliminate the inflammatory response from them helping nerve cells survive

6. Granulocyte-macrophage colony-stimulating factor (GM-CSF) – expose old microglial cells to this and conditioned media of young macrophages helped induce microglial proliferation and reduced Aβ levels

7. Acucanumab – a monoclonal antibody that targets aggregated Aβ, reduces soluble and insoluble Aβ in a dose-dependent manner

All in all, finding a cure for AD will take a lot more research and experimental trials. Hopefully, though, some day there will be a break through and will save many people from the unfortunate symptoms of the disease.
Clearance of Beta-Amyloid Plaques in Alzheimer’s Disease: The Key to the Cure?