What are nootropics
Nootropics are synthetic molecules marketed towards enhancing cognition and increasing focus. There are currently no over the counter nootropics that are FDA approved. Human cognition is a complex and intricate pathway that cannot easily be altered on demand. Therefore, many over the counter nootropics may be acting more as a placebo.
Cognition
The DSM-5 outlines six neurocognitive domains that are linked to an increase in learning and memory which are: perceptual and motor function, language, learning and memory, social cognition, attention, and executive functions.
Perceptual and motor function: visual and visuo-constructional reasoning along with motor coordination.
Language: syntax, grammar, object naming, receptive language, and verbal fluency.
Learning and memory: semantic and episodic memory along with implicit learning. Social cognition: Recognition of individuals emotions and intentions.
Attention: Processing speed and sustained attention.
Executive function: Working memory, planning, decision-making, and flexibility.
These domains do not work independently but rather as an integrated system. Therefore, to effectively enhance cognition a nootropic would have to alter all six pathways.
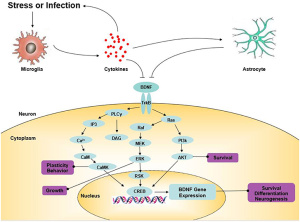
How nootropics work
Nootropics work by positively modulating AMPA receptors and increase neurotrophin levels in the brain. Ampakines are a class of nootropics that are small molecules the bind and open AMPA receptors to allow for the influx of ions. Ampakines are derived from the nootropic aniracetam. The ampakine binding slows receptor deactivation and desensitization rates. The increased length of the receptor being open allows for a rapid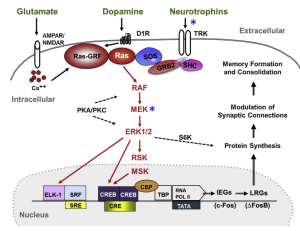 increase in excitatory transmission and ultimately lowers the threshold for long term potentiation (LTP). LTP aids in learning and memory so by lowering this threshold learning can occur at a faster rate. Ampakines can additionally upregulate BDNF expression. Increases in BDNF can surpass the drugs half-life and continue supporting neuronal differentiation and survival. An increase in BDNF can also lead to the increased activation of the Ras-ERK pathway. The Ras-ERK pathway is implicated in establishing long term memories and plasticity. Ampakines can be categorized as either high impact or low impact. High impact ampakines slow the AMPA channels from closing and low impact ampakines accelerates the AMPA channel opening.
increase in excitatory transmission and ultimately lowers the threshold for long term potentiation (LTP). LTP aids in learning and memory so by lowering this threshold learning can occur at a faster rate. Ampakines can additionally upregulate BDNF expression. Increases in BDNF can surpass the drugs half-life and continue supporting neuronal differentiation and survival. An increase in BDNF can also lead to the increased activation of the Ras-ERK pathway. The Ras-ERK pathway is implicated in establishing long term memories and plasticity. Ampakines can be categorized as either high impact or low impact. High impact ampakines slow the AMPA channels from closing and low impact ampakines accelerates the AMPA channel opening.
Current research
Research thus far has looked at the possibilities of nootropics in proving cognition in older adults and those suffering from Huntington’s and Alzheimer’s disease. The main goal has been to find ways to slow the cognitive decline associated with these diseases. Therefore, researchers recommended young and healthy individuals refrain from taking nootropics since studies have not looked at the effects of nootropics on that age range.
Conclusion
Nootropics or “smart drugs” are thought to enhance cognition and aid an individual in focus and learning. However, human cognition is comprised of six complex and intertwined domains. Therefore, to enhance cognition would be to activate all six domains. Researchers caution against taking over the counter nootropics since they are not FDA approved and have little scientific study of them. Studies that have been done have looked at the nootropic class of ampakines and how they can help older adults’ cognition and those diagnosed with Huntington’s and Alzheimer’s disease.
References
[1] Morè, L., Lauterborn, J. C., Papaleo, F., & Brambilla, R. (2020). Enhancing cognition through pharmacological and environmental interventions: Examples from preclinical models of neurodevelopmental disorders. Neuroscience & Biobehavioral Reviews, 110, 28–45. https://doi.org/10.1016/j.neubiorev.2019.02.003
[2] Brody, Barbara (2022). What are nootropics? WebMD. https://www.webmd.com/vitamins-and-supplements/features/nootropics-smart-drugs-overview
[3] RespireRX (2017). Ampakines development summary. https://www.sec.gov/Archives/edgar/data/849636/000149315217007695/ex99-3.htm