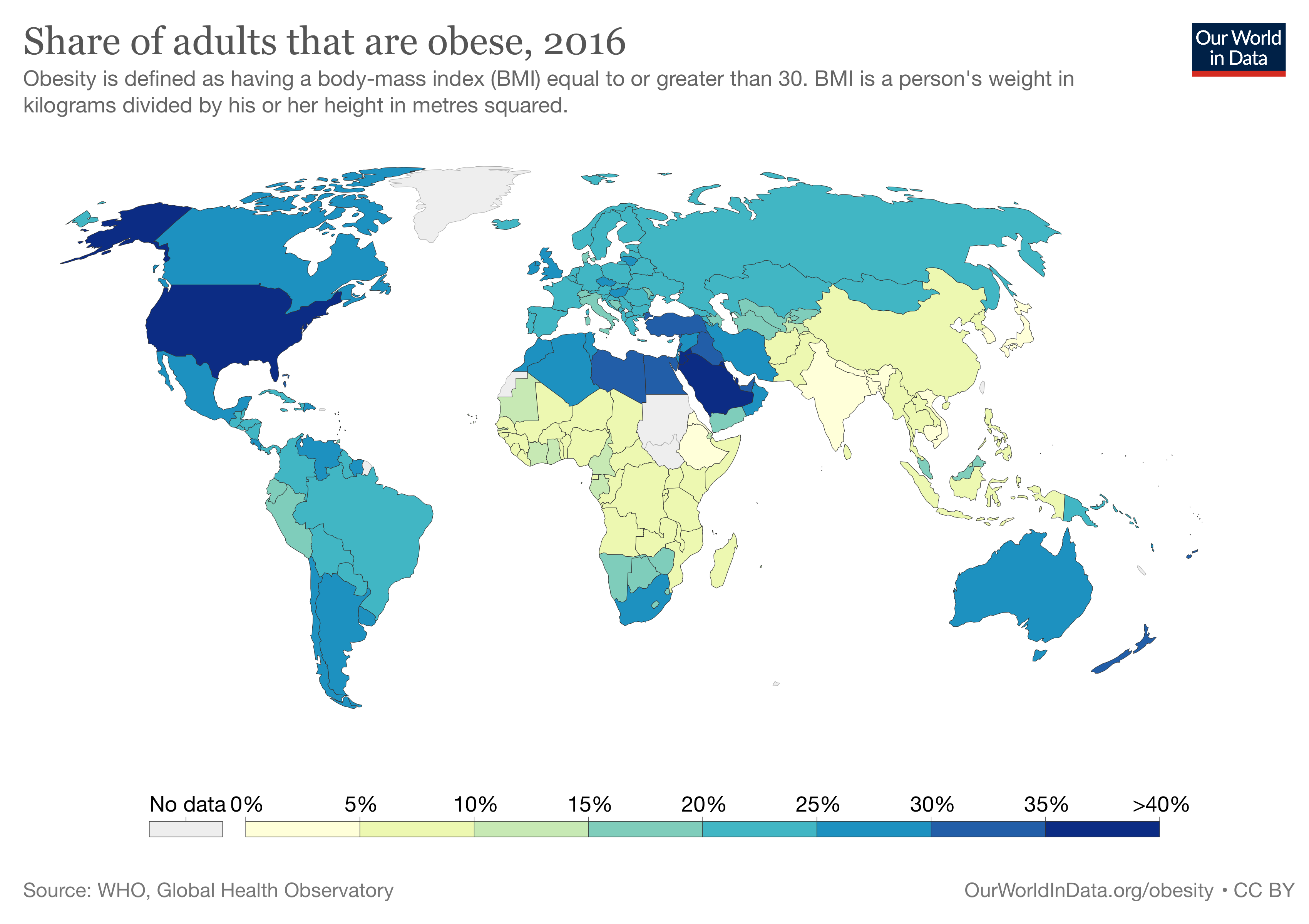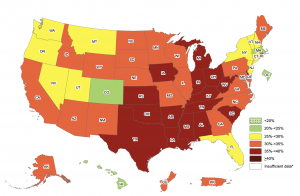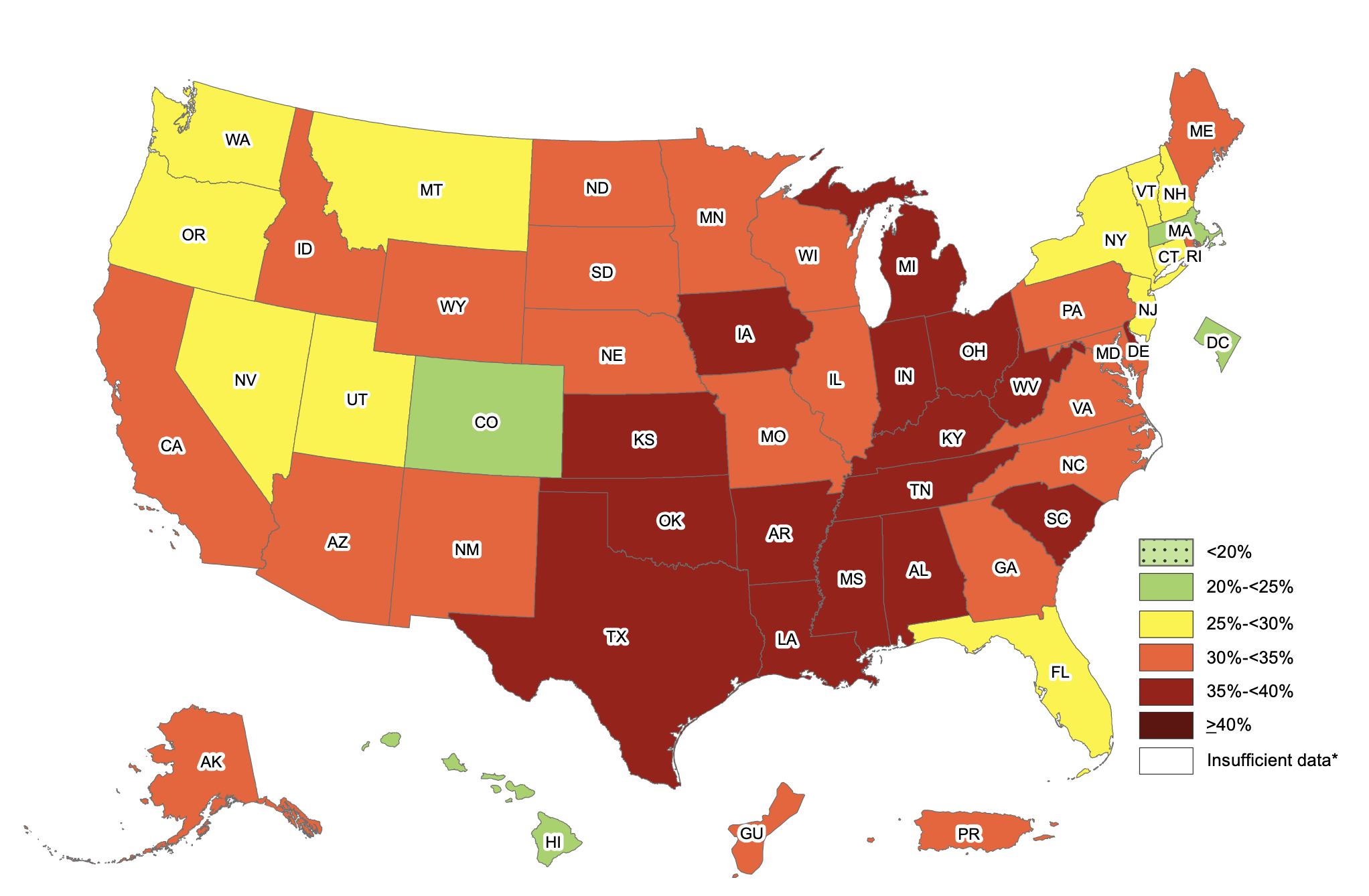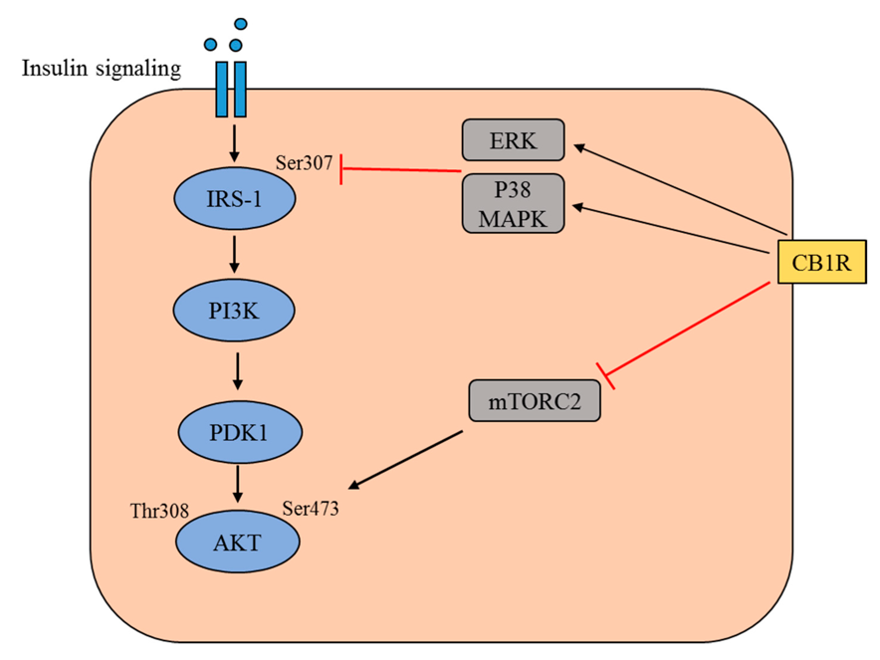
The obesity rate within America during 2017-2018 sat at a staggering 42.4%. If that wasn’t bad enough, 73.6% of Americans are now considered to be overweight. Look at the chart below, obesity rates within the US have increased exponentially since the 60’s. Several drastic changes occurred during the 80’s within American culture/society that impacted obesity rates, leading to the exceptionally high rates of obesity.
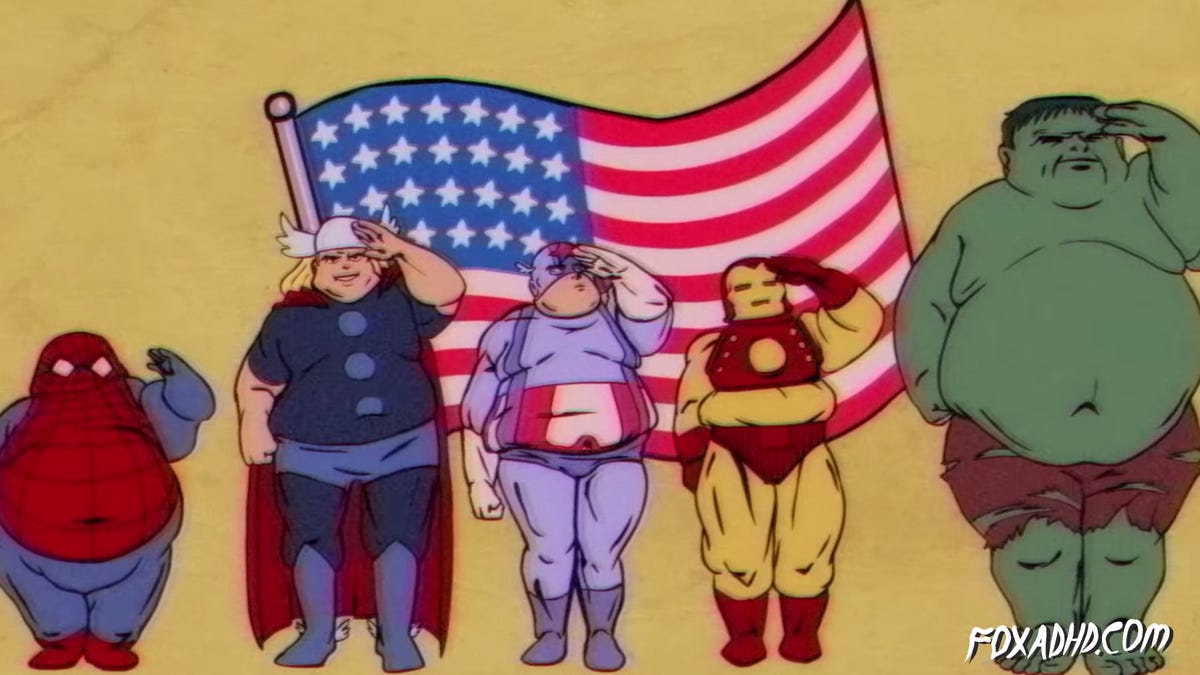
Obesity is associated with many negative health conditions. These conditions cost America anywhere between 147 and 210 billion dollars annually. Will our societal eating habits become so greatly problematic to the point of Wall-E? As a kid I thought the movie was funny, the portrayal of the overweight people seemed to be amusing and unrealistic since there appeared to be a complete lack of healthy humans. However, if Americans continues this trend towards increased obesity, we could end up looking similar to those pictured in this fictional movie. Would I still find it funny, probably not.
Picture credits; Wall-E
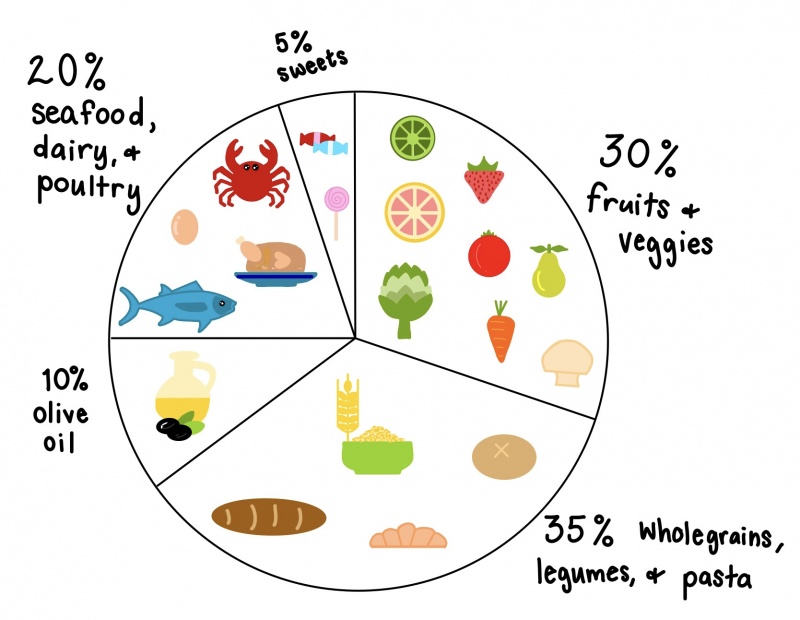
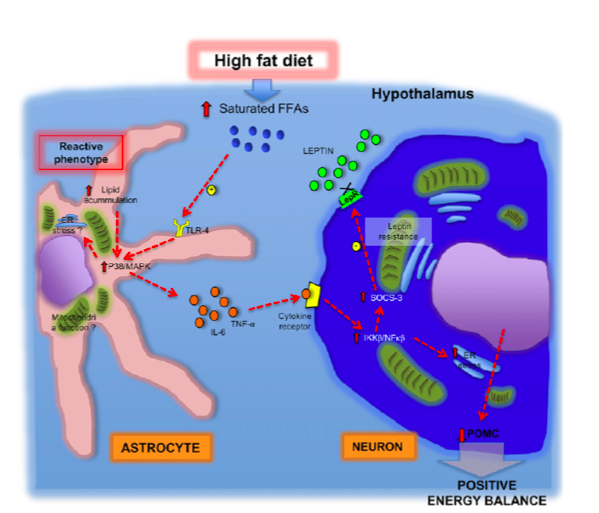
One of the changes mentioned earlier that took place in the 80’s relates to the change in meal composition. As a society we are moving away from healthier meals in favor of high fat diets. We talked at length about this in class, there are several negative consequences of eating a high fat diet. You can find that information on this document if you would like to look more into it. As an example of how we’ve changed out consumption of different foods, lets look at a couple of examples. Consumption of vegetable oil within Americans diets has DRASTICALLY increased over time. 400 additional calories are consumed daily due to this one ingredient alone. Unfortunately that is not the only food “category” that has changed. Look below to see examples of three food categories and how their average (daily) caloric intake has changed over time.
Here is a list of the top calorie sources within the US diet
1. Grain-based desserts (like cakes, cookies, and donuts)
2. Breads made with yeast
3. Chicken and chicken dishes
4. Soda and sports drinks
5. Pizza
6. Alcoholic beverages
7. Pasta and pasta dishes
8. Mexican dishes
9. Beef and beef dishes
10. Dairy desserts (like ice cream and cheesecake)
In case you hadn’t noticed, none of these are overly healthy options and are all very high in fat, leading back to the article relating to high fat diets and how unhealthy that is for us.
The American diet in terms of daily calories has changed over time. Today Americans consume more than 3,600 calories every day which is a 24% increase from 1961 when Americans only consumed 2,880 calories daily. The chart below shows how the US food chain has ballooned over time in order to meet the demands of the consumers. The chart shows slightly different estimates of daily caloric intake, estimates that vary per source, but it shows the shift from 2,880 to 3,600 calories. Sources seem to have conflicting estimates, but almost all seem to agree that daily caloric intake has risen by at least 20%.
What changes have lead Americans to become so obese, especially the large spike in obesity during the 80’s? Well the simple answer includes several things: a change in the composition of meals (for example more saturated fat and vegetable oil), source of food (fast food over home cooking), and lifestyle changes (less exercise and more loungin around watchin the Vikings lose).
In terms of a changing food source, the graph below does a good job showing where families have gotten their food from over the years. Families used to eat at home, now fast food and restaurants compose a much larger percentage of weekly meals.
Unfortunately obesity in America is not only a problem of consumption, it is also a problem of physical exertion. What do I mean by this? Well, most Americans fail to meet the US governments minimum daily recommended exercise. That recommendation includes 75 minutes of vigorous cardiovascular exercise a week as well as muscle strengthening twice a week. Sadly only 23% of Americans achieve this minimal standard. Time is spent doing other things, for example watching television.
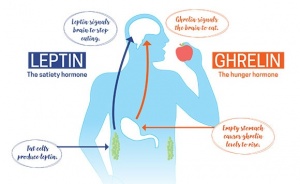
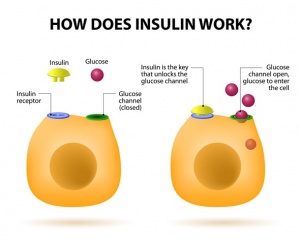
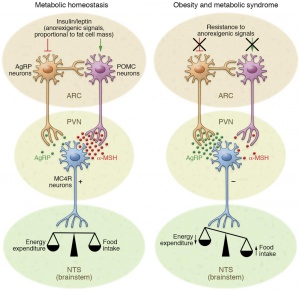
With Americans having a higher caloric intake and a decrease in exercise it is not shocking to see a drastic rise in obesity. Lets all get out of our chairs, eat a little better, and work as a society to better our health. If we don’t and our diets continue to contain a high ratio of fat, we might get to a point where our body becomes resistant to both insulin and leptin. This scenario would lead to a detrimental feedback loop that would benefit no one, leading to even higher rates of obesity with little hope for individual health and well being.
Here’s a link to a video of me explaining my topic if you’d rather listen to my voice instead of reading: https://drive.google.com/file/d/1W9P1wZxyiVZsQILe2YTxtd4Sm4rKFIo-/view?usp=sharing
Centers for Disease Control and Prevention. (2021, September 30). Adult obesity facts. Centers for Disease Control and Prevention. Retrieved November 16, 2021, from https://www.cdc.gov/obesity/data/adult.html.
Centers for Disease Control and Prevention. (2021, September 10). FastStats – overweight prevalence. Centers for Disease Control and Prevention. Retrieved November 16, 2021, from https://www.cdc.gov/nchs/fastats/obesity-overweight.htm.
Schuler, L. (2021, February 12). The real reason Americans are so fat. Men’s Health. Retrieved November 16, 2021, from https://www.menshealth.com/weight-loss/a19536794/reasons-americans-are-fat/.
” why the world is overweight? the 1980’s and its contribution to our health now! Size Fantastic – Sustainable Weight Control – Healthy Weight Control and Weight Loss. (n.d.). Retrieved November 16, 2021, from https://sizefantastic.com.au/weight-loss-tips/why-the-world-is-overweight-the-1980s-and-its-contribution-to-our-health-now/.