What is Autism?
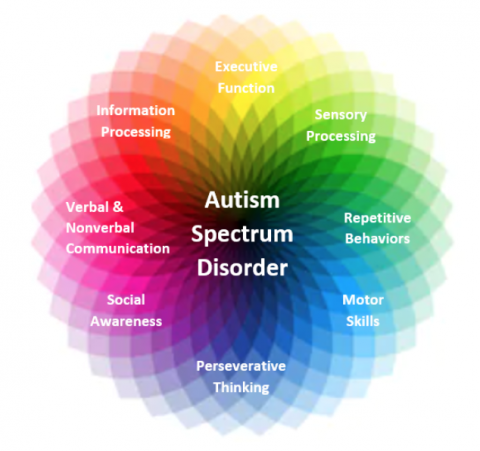
- Autism (ASD, Autism Spectrum Disorder) is a neurodevelopmental, lifelong condition, with a broad range of conditions characterized by challenges faced by an individual. There are many social skills, repetitive behaviors, speech and nonverbal communication involved with autism. However, each person with autism is unique and may have better or worse skills.
- An ASD diagnosis now includes other conditions that used to be diagnosed separately, such as Asperger syndrome and pervasive developmental disorder
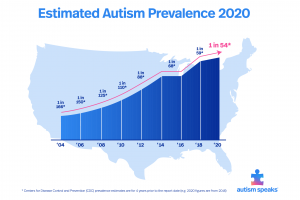
- Autism begins to develop during pregnancy and goes through the first few years of a child’s life. If proper nutrition is not available to the mothers during pregnancy, there is an increased risk of having a child with autism. Some nutrients such as zinc, vitamin D, and omega-3 fatty acids have been linked to neuropsychological symptoms. Low zinc has been linked to learning and cognitive impairments, while low vitamin D has been linked to less aid with brain function and axonal connectivity. During the first few years of life, there is abnormal overgrowth in the cerebral, cerebellar, and limbic structures. The overgrowth happens in regions associated with cognition, social and emotional functions, as well as language functions. This overgrowth is then followed by an abnormally slow or stunted growth.
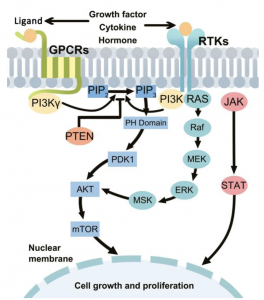
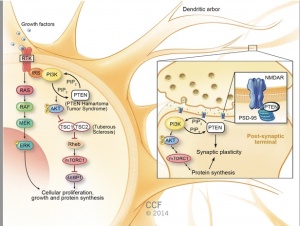
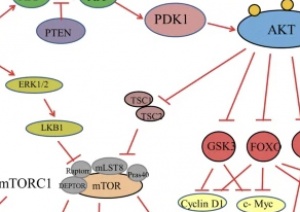
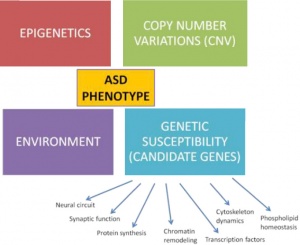
PI3K/ AKT-(PKB)/ mTOR pathway:
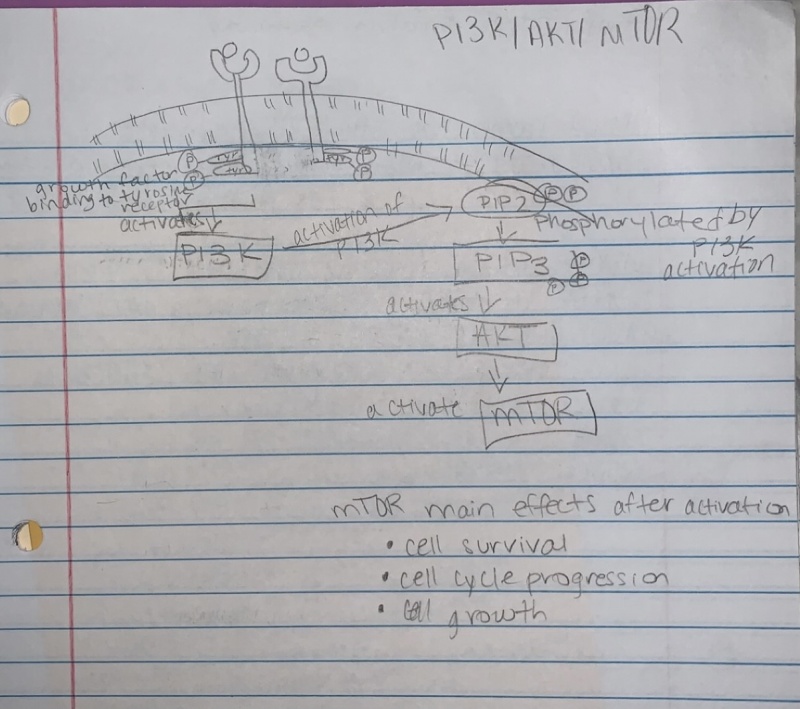
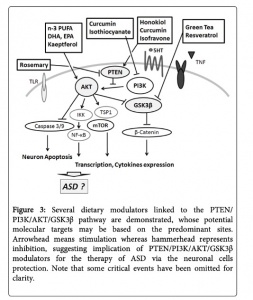
- Overactive PI3k/Akt/mTOR pathway in autism. See image to the right for pathway.
- The dysfunctional PI3K/Akt/mTOR pathway is involved in many processes that are related to the autism spectrum disorder. These include apoptosis, the deficit in social interaction, repetitive behavior, and hyperexcitability.
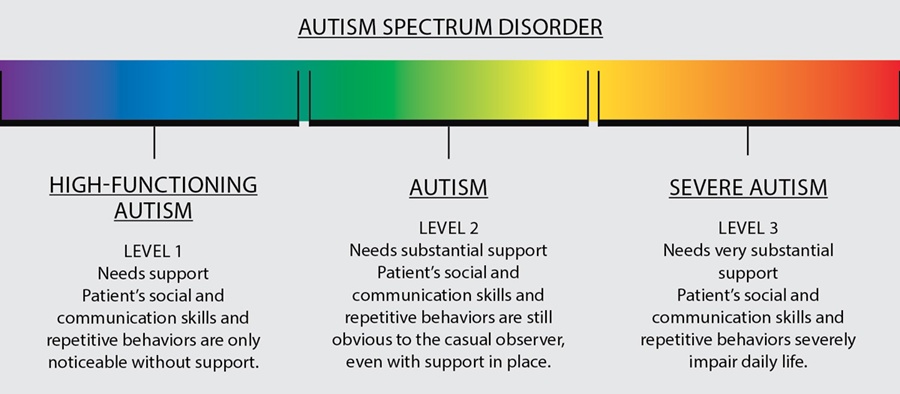
Symptoms of Autism: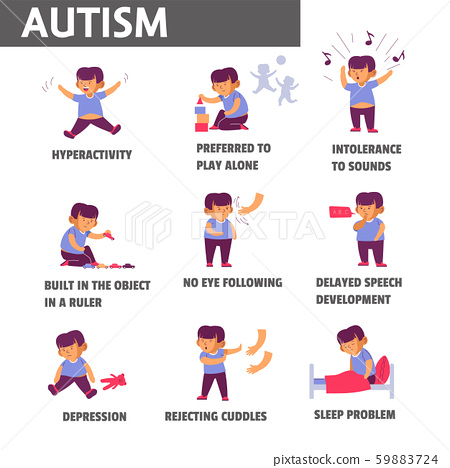
- It is important to understand that many individuals with autism do not experience all of these symptoms, or experience ones that are not listed. Also, individuals with autism who do experience many symptoms do not experience them the same way as others with autism.
- Symptoms of autism may include:
- Lack of social communication and interactions
- Avoids or cannot maintain eye contact
- Lack of play, especially social play
- Lack of communication with peers
- Uses few or no gestures
- Difficulty understanding own feelings and the feelings of others
- Restricted or repetitive behaviors or interests
- May repeat words or phrases
- Uses toys the same way
- Focused more on the parts of the object, rather than the object as a whole
- Certain routines must be followed daily
- Can flap hands, rock body, etc
- Other possible characteristics of Autism
- Delayed language, cognitive, and movement skills
- Hyperactive, impulsive
- Unusual eating and sleeping patterns
- Anxiety and excessive worry about situations
Possible Treatments of Autism:
- Autism has no cure, but there are therapies that may help manage symptoms and allow individuals to be “higher functioning”
- Therapies to help individuals:
- Occupational therapy
- Speech therapy
- Pivotal response treatment (PRT)
- Relationship development intervention (RDI)
- Comorbidities that require medical treatment, not only therapies:
- Epilepsy
- Gastrointestinal problems
- Sleep disturbances
- Attention-deficit/hyperactivity disorder
- Anxiety/ Depression
- Obsessive-compulsive disorder
Works Cited:
Autism Speaks Inc. (2021). Treatments for autism. Autism Speaks. Retrieved November 8, 2021, from https://www.autismspeaks.org/treatments-autism.
Centers for Disease Control and Prevention. (2021, March 29). Signs and symptoms of autism spectrum disorders. Centers for Disease Control and Prevention. Retrieved November 8, 2021, from https://www.cdc.gov/ncbddd/autism/signs.html.
Sharma, A., & Mehan, S. (2021). Targeting PI3K-AKT/mTOR signaling in the prevention of autism. The Journal of Cellular and Molecular Neuroscience.







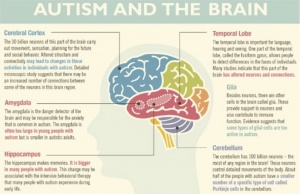
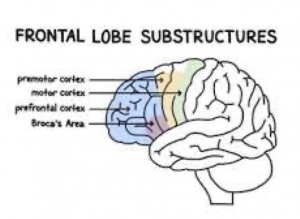 frontal cortex in children and adolescents with ASD are significantly enlarged. The frontal cortex is responsible for managing attention, understanding the feelings of other people, speech and language production, and forming one’s personality. Abnormalities in the frontal cortex associated provide
frontal cortex in children and adolescents with ASD are significantly enlarged. The frontal cortex is responsible for managing attention, understanding the feelings of other people, speech and language production, and forming one’s personality. Abnormalities in the frontal cortex associated provide 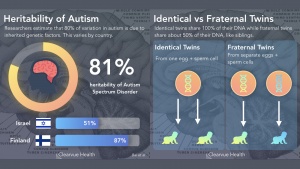


:max_bytes(150000):strip_icc()/treatments-for-adults-with-asperger-syndrome-259901-Final-1383adbeffcc4d3a9a2c7020d7bb306d.png)
![PDF] Drug therapy in autism: a present and future perspective. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/145fda4d09aa46a5e6dc9685253313d808fafb73/5-Figure1-1.png)