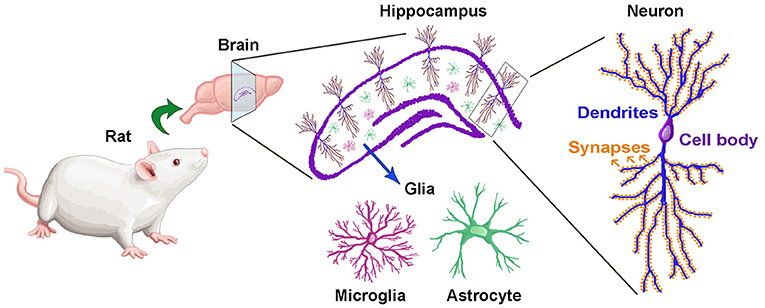
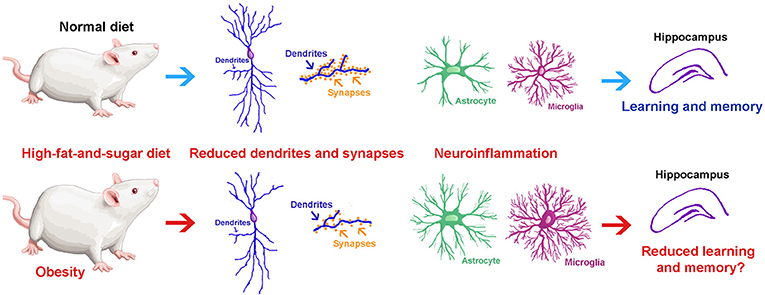
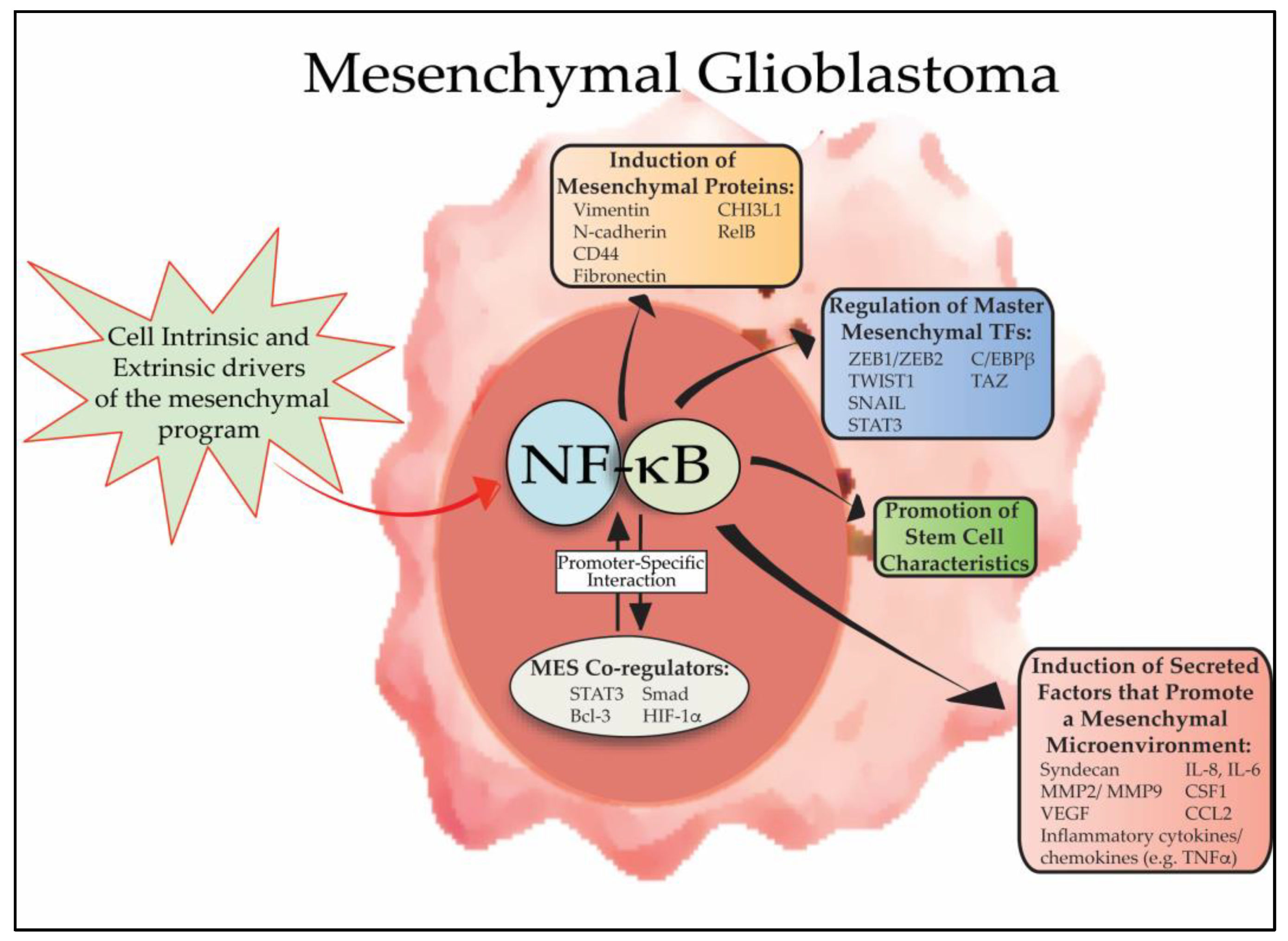
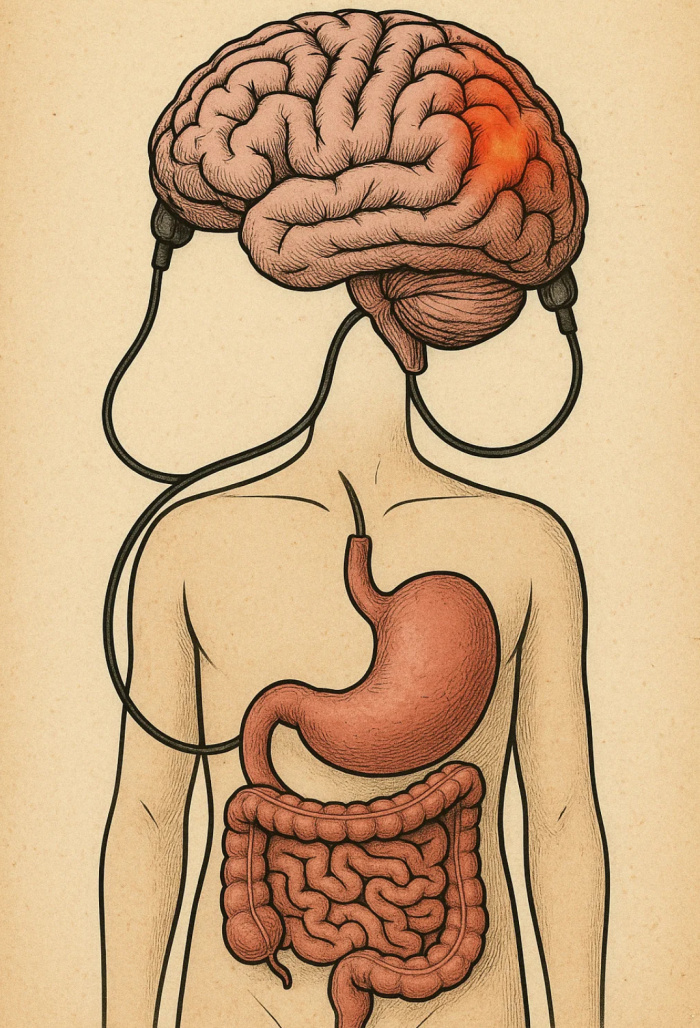
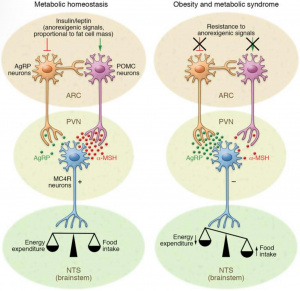
Every ending is also a beginning — and as I close this chapter at Concordia, I find myself reflecting on how this journey has shaped who I am and how I see the world. As I reflect on my experience in this course and my broader education at Concordia, I realize how much the CORE curriculum and the philosophy of liberal learning — to Become Responsibly Engaged in the World (BREW) — have truly shaped my academic and personal growth. This class served as a culmination of the many skills, competencies, and perspectives I have gained during my time at Concordia, allowing me to not only apply what I have learned across disciplines but also to better understand my role in the larger global community.
Throughout the semester, the knowledge I gained from participating in this class was both academic and personal. Academically, I strengthened foundational skills such as critical thinking, comprehension and processing of research articles in a variety of fields, communication, and collaboration. Each of our weekly assignments not only made me better understand the topics discussed in each of the articles, but also it challenged me to think beyond surface-level understanding, encouraging me to connect theoretical knowledge to real-world applications. Personally, the group discussion aspect of this course pushed me to reflect more deeply on my values, my approach to problem-solving, and my ability to engage with diverse perspectives. Learning, for me this semester, was an active process of integration — weaving together threads from different courses, disciplines, and experiences into a cohesive understanding of complex issues.
The skills and competencies I gained this semester are directly aligned with my future goals. As I look toward a career in the field of chemistry that requires not only technical knowledge but also the ability to think critically, adapt, and lead ethically, I am grateful for the emphasis Concordia has placed on transferable skills. Whether it is analyzing data, finding and researching scientific topics of interest, creating stories and communicating them in a way that fit my audience, navigating intercultural communication, or leading a team with empathy and responsibility, the liberal learning goals have prepared me to meet these challenges. In particular, developing interdisciplinary perspectives has been invaluable. Problems in the real world are rarely isolated within one field, and my education has trained me to draw from multiple disciplines to find creative and effective solutions.
Learning at a liberal arts institution like Concordia has meant more than simply mastering content; it has meant developing a mindset of lifelong curiosity and responsibility. It has taught me to ask not just “How?” but also “Why?” and “For whom?” Concordia’s commitment to cultivating an examined self — culturally, ethically, physically, and spiritually — has encouraged me to be mindful of the broader impact of my actions. It has challenged me to think about my place in society and to recognize my responsibility to contribute positively to my community and beyond. In a world that is increasingly complex and interconnected, I believe this kind of education is more important than ever.
If I were to highlight a few skills I really strengthened this semester, I would definitely focus on communication, research and information literacy, and time management. Over the past few months, I had so many opportunities to practice different forms of communicating clearly — whether it was writing scientific based papers or blog posts, giving presentations, or simply explaining my thoughts and ideas in class discussions. I learned that good communication isn’t just about sharing information; it’s about telling a good story and making sure your message connects with your audience, and that’s something I know I’ll carry into my future work. I want to specifically highlight the opportunities that I have had to communicate in a public speaking manner. Public speaking or just clearly communicating my thoughts out loud to any size of audience has always been a struggle for me. I have gone through a lot of speech therapy throughout my life, especially in my childhood, and so public speaking is scary and difficult for me as I usually revert back to bad habits that make it hard to understand what I am trying to say. I have had plenty of practice with this over the years, but I think in this class as well as through senior chemistry seminar has been extremely influential in helping me feel more confident in my abilities and has given me hope that I can give solid scientific talks in the near future.
This semester also pushed me to become much more confident in my research skills. Finding reliable sources, evaluating information critically, and weaving different perspectives into a clear argument has become almost second nature. I realized how important it is not just to find information, but to understand and use it responsibly — a skill that’s incredibly valuable in any career path.
And finally, managing all the moving pieces of this semester really tested — and improved — my organizational skills. Being my fifth and final year here at Concordia came with some exciting opportunities to show off what I have done and learned such as senior chemistry seminar. This added to the process of balancing academic deadlines, looking for my next career step, and other outside commitments which forced me to plan ahead, stay focused, and adjust when things didn’t go exactly as planned. I struggled a lot with staying healthy this and last semester which really took me out of the flow that I have gotten used to being in during college. The previous semester showed it in terms of my academic performance and my struggles with bouncing back from being behind. However, I think I was able to learn from that experience, and even though I was dealing with similar issues this semester, I handled the situation much better and got back into the swing of things much more efficiently. I can honestly say I’m ending this semester more confident in my ability to stay organized and handle competing priorities, which will definitely help me moving forward.
Overall, this course — and my education at Concordia more broadly — has instilled in me a deep love for learning, an ability to think across disciplines, and a commitment to being a responsible, engaged participant in the world. I now see liberal learning not just as an academic ideal but as a lifelong practice — one that calls me to continue growing, questioning, and contributing, wherever my future may take me.