Bigger Isn’t Better: Inflammation Affects on The Brain
Swelling of the brain might seem like a minor concern when it comes to overall brain health, but inflammation affects homeostasis, disrupting processes such as hunger regulation and metabolism. Hypothalamic inflammation leads to issues such as overeating, energy imbalance, and systemic health issues like diabetes and heart disease. Inflammation is the silent driver of metabolic dysfunction that produces diabetes.
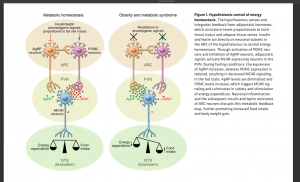
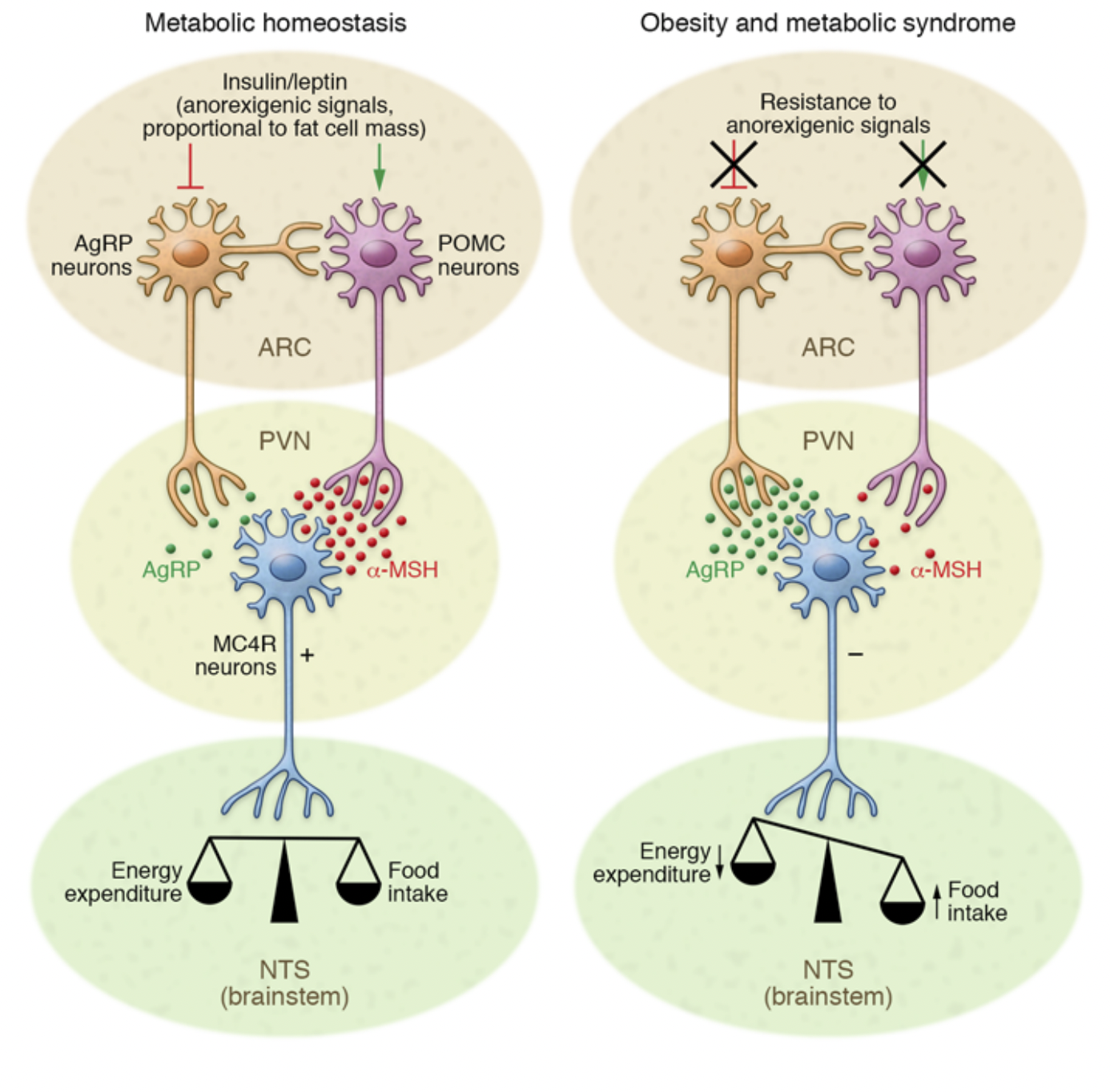
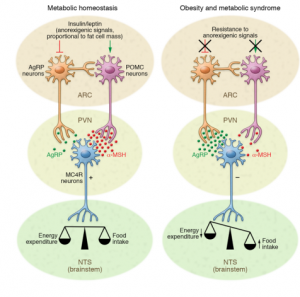
Low-grade inflammation is often caused by obesity, which weakens signaling and disrupts metabolic homeostasis. This type of signaling is labeled as anorexogenic signaling which contributes to fat cell mass and has an affect on the relationship between food intake and energy expenditure. As shown in Figure 1., when anorexigenic signaling decreases, orexigenic signal increases which signals the body to increase appetite and food intake. On the other hand, anorexigenic signaling lowers food intake. Diet also plays a significant role in inflammation. A high fat diet (HFD) or low fat diet (LFD) can affect this signaling in a positive or negative way.[1]
How Hypothalamic Inflammation Develops
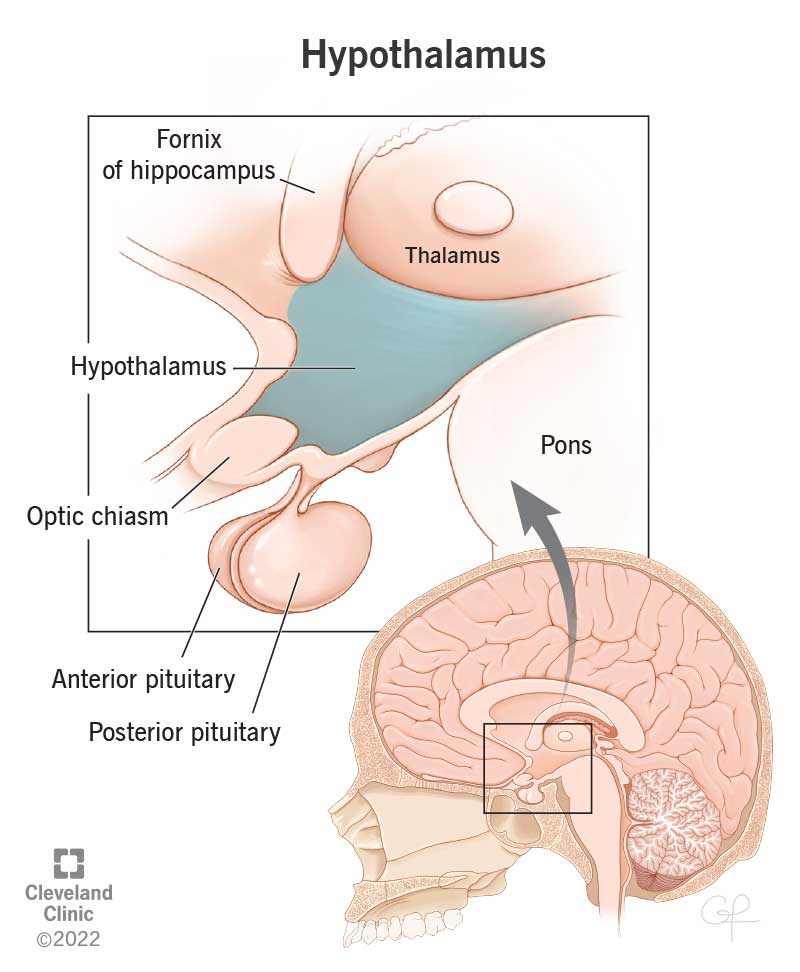
Hypothalamic inflammation is chronic inflammation that affects the hypothalamus region the most, this region is in charge of regulating energy, balance and bodily functions that contribute to homeostasis.
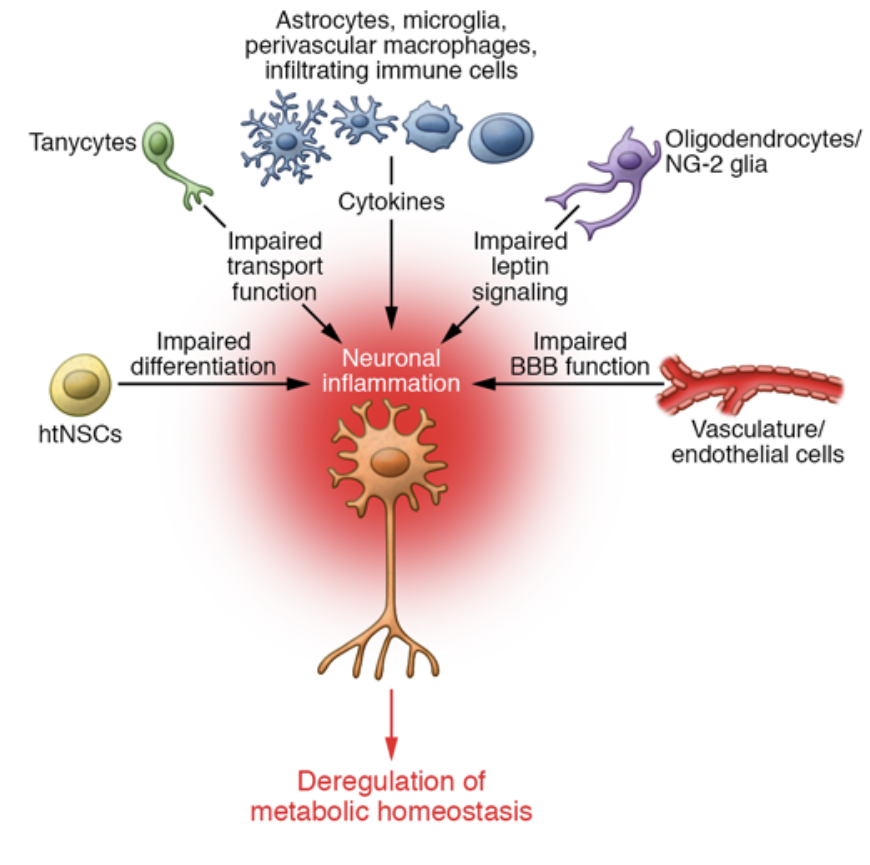
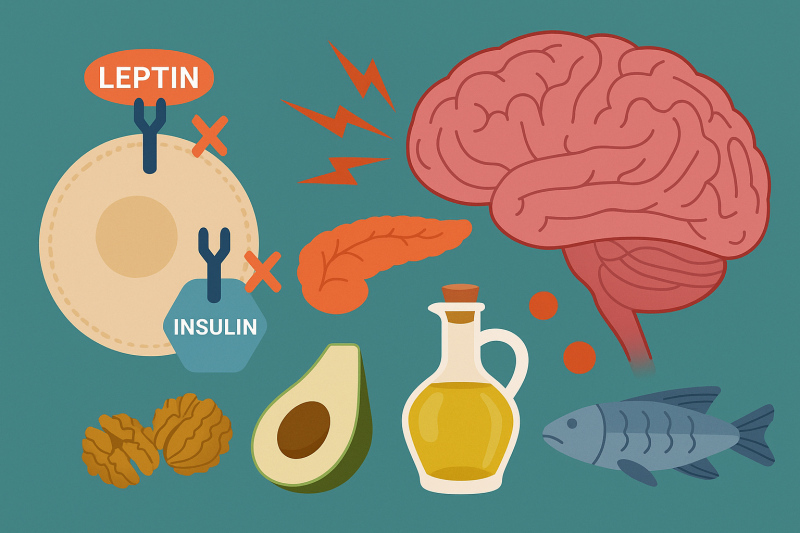
Two hormones, Insulin and Leptin, are major players in how hypothalamic inflammation develops. Leptin is a hormone that is secreted by adipose tissue, AKA body fat, which stores energy. Leptin contributes to Insulin resistance; elevated levels of Leptin are typically due to an increased fat mass. Insulin resistance interferes with Leptin’s normal function: maintaining energy by suppressing hunger. Leptin’s job is to signal to the brain when energy storage is in primal conditions. When Leptin contributes to Insulin resistance it disrupts the glucose metabolism and promotes fat accumulation. There are a multitude of diseases that contribute to hypothalamic inflammation as shown in Figure 2.

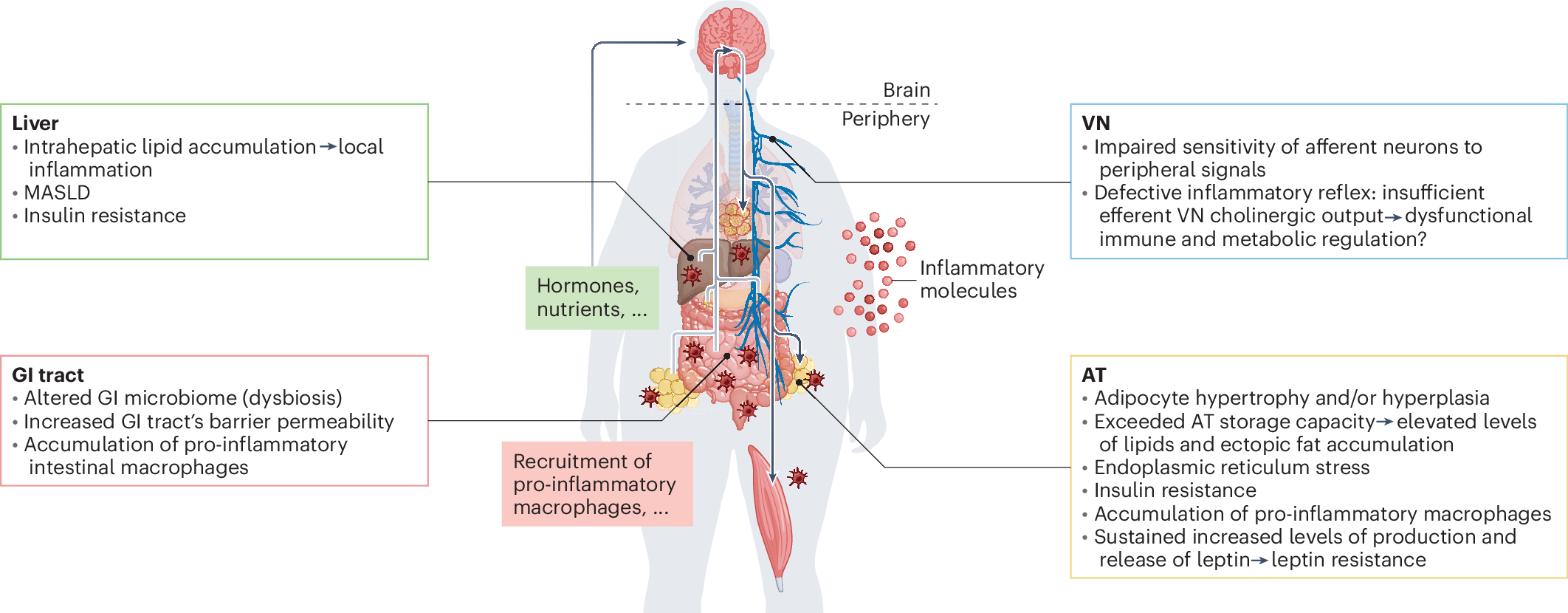
The blood–brain barrier (BBB) acts as security, regulating the transport of the metabolic signals we receive from the central nervous system (CNS). Disruptions in BBB function can contribute to metabolic and neurological disorders, such as obesity and metabolic syndrome. Elevated triglyceride levels have been shown to impair Leptin transport across the BBB, inducing peripheral Leptin resistance and weakening the brain’s ability to regulate energy homeostasis. Similarly, chronic inflammation and Insulin resistance can alter theBBB strength, affecting cognitive functioning and increasing the chance of neurodegenerative diseases such as Alzheimer’s.[2]
This type of systemic metabolic dysfunction contributes to cognitive and neurological impairments, highlighting the need for targeted interventions to restore metabolic balance and brain health.
Chronic inflammation in the hypothalamus plays a critical role in metabolic dysfunction. There are two main signaling pathways involved:
c-Jun N-terminal kinase (JNK)
IκB kinase (IKK)
These kinases are activated in response to metabolic stress, including excessive nutrient intake, obesity, and Insulin resistance. JNK activation interferes with Insulin signaling by phosphorylating IRS-1, a key mediator in the Insulin pathway, contributing to Insulin resistance and Leptin dysfunction.
Similarly, IKK plays a pivotal role in activating NF-κB, a transcription factor that drives inflammatory responses, further disrupting metabolic homeostasis and impairing brain function.
Elevated activity of these pathways not only exacerbates hypothalamic inflammation but also impairs blood-brain barrier integrity, influencing cognitive function and neurodegenerative disease risk. Understanding how JNK and IKK contribute to metabolic inflammation offers valuable insight into the mechanisms driving obesity, Insulin resistance, and associated neurological disorders.
YUM! Saturated Fatty Acids
We have a general idea of what foods are unhealthy versus heathy. What we choose to put in our bodies develops habits that determine our overall health. It is important to treat your body well and keep your body happy. Studies show that the influence diet has affects not only your body, but also your brain. The response is negative or positive based on what you feed it.
LFD
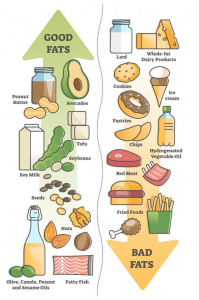
A low-fat diet (LFD) prioritizes reducing saturated fats, AKA your dietary fat, as shown in Figure 3. While recommended for heart health and weight management, newer research suggests that fat quality matters more than quantity. Some fats, like omega-3 fatty acids, support brain health, while excessive intake of unhealthy fats can promote inflammation and Insulin resistance as shown is Figure 4.

Acute HFD
Short-term exposure to a high-fat diet (HFD) has been shown to disrupt normal metabolic processes, leading to temporary Insulin resistance and increased inflammatory markers. In animal studies, acute HFD exposure has been linked to impaired Leptin signaling, weakening the brain’s ability to regulate hunger and energy balance.
Chronic HFD
Long-term consumption of a high-fat diet presents more severe consequences. Chronic HFD has been extensively studied for its role in promoting obesity, metabolic dysfunction, and neuroinflammation. Continued exposure to excessive saturated fatty acids contributes to the risk of hypothalamic inflammation, disrupting Leptin and Insulin signaling, impairing the BBB strength, and increasing the risk of neurodegenerative diseases. [3]

The Melanocortin System and Energy Regulation
The melanocortin system is involved in various physiological processes, including energy balance, immune regulation, and pigmentation. This complex network comprises melanocortin peptides derived from pro-opiomelanocortin (POMC), five melanocortin receptors (MCRs), and two endogenous antagonists—agouti-signaling protein and agouti-related peptide. Research has discovered the importance of this system and its broader influence on inflammation, metabolic regulation, and neural signaling.
The melanocortin system consists of three important players: POMC, AgRP, and FOXO1 neurons.
POMC neurons
The activity of POMC neurons is tightly regulated by various signals, including Leptin and Insulin, which contributes to promote satiety. POMC neurons are functionally opposed by AgRP neurons, which exert antagonistic effects on melanocortin signaling by inhibiting MC3R and MC4R activity, promoting hunger and reducing energy expenditure.[4]
AgRP neurons
Agouti-related peptide (AgRP) is primarily involved in regulating appetite, energy balance, and promoting food intake. Melanocortin receptors, MC3R and MC4R, take part in metabolism and food intake. By binding to these receptors, AgRP effectively suppresses melanocortin signaling, leading to increased feeding behavior and reduced energy expenditure, as shown in Figure 5.
FOXO1
AgRP’s role in metabolism and energy balance is closely linked to transcription factors that regulate cellular processes, including FOXO1. FOXO1 (Forkhead box protein O1) is a key transcription factor involved in Insulin signaling, gluconeogenesis, AKA the process where the liver and kidneys make sugar, and neuroendocrine regulation.
When energy levels are low, FOXO1 becomes more active and enhances AgRP gene transcription, promoting appetite stimulation and increasing food intake. This interaction is crucial in energy deficient states, such as fasting or caloric restriction, where the body prioritizes nutrient intake and conservation. Conversely, Insulin signaling inhibits FOXO1 activity, reducing AgRP expression and suppressing hunger.[5]

The Brain is #1
The brain is the most important part of our bodies. It is responsible for everything that we are able to do. Protecting our brain and prioritizing brain health means making healthy choices that support our metabolic balance and minimize inflammation. A high-fat diet, when sustained over time, not only leads to obesity but also fuels Insulin resistance and inflammatory processes that compromise brain health. This type of damage can be avoided with lifestyle changes by prioritizing anti-inflammatory nutrients.We can improve metabolic flexibility by choosing whole, nutrient-dense foods. By making healthy choices, we set a foundation for long-term physical and cognitive resilience.
REFRENCES
Banks WA, Farr SA, Salameh TS, Niehoff ML, Rhea EM, Morley JE, Hanson AJ, Hansen KM, Craft S. Triglycerides cross the blood-brain barrier and induce central leptin and insulin receptor resistance. Int J Obes (Lond). 2018 Mar;42(3):391-397. doi: 10.1038/ijo.2017.231. Epub 2017 Oct 9. PMID: 28990588; PMCID: PMC5880581.
Henn, R. E., Elzinga, S. E., Glass, E., Parent, R., Guo, K., Allouch, A. M., Mendelson, F. E., Hayes, J., Webber-Davis, I., Murphy, G. G., Hur, J., & Feldman, E. L. (2022). Obesity-induced neuroinflammation and cognitive impairment in young adult versus middle-aged mice. Immunity & ageing : I & A, 19(1), 67. https://doi.org/10.1186/s12979-022-00323-7
Jais, A., & Brüning, J. C. (2017, January 3). Hypothalamic inflammation in obesity and metabolic disease. The Journal of clinical investigation. https://pmc.ncbi.nlm.nih.gov/articles/PMC5199695/
Kentish, S.J. and Page, A.J. (2015), The role of gastrointestinal vagal afferent fibres in obesity. J Physiol, 593: 775-786. https://doi.org/10.1113/jphysiol.2014.278226
Wang, W., Guo, D. Y., Lin, Y. J., & Tao, Y. X. (2019). Melanocortin Regulation of Inflammation. Frontiers in endocrinology, 10, 683. https://doi.org/10.3389/fendo.2019.00683