Alzheimer’s Disease (AD) is one of the most saddening diseases. AD not only affects the patient, it could wreak emotional havoc on the family and friends of the patient. AD is the most common cause of dementia among older adults1. Dr. Alois Alzheimer was the first to diagnose this wretched disease after a postmortem study. He noticed changes in the brain tissue of a woman who had died of an unusual mental illness in which her symptoms included memory loss, language problems, and unpredictable behavior. The postmortem examination found that she had many abnormal clumps (amyloid plaques) and tangled bundles of fibers (neurofibrillary tangles)1. To this day, these plaques and tangles are still the main features of AD’s pathogenesis. What causes these plaques and tangles to form? I was surprised to find that the answer is closely related type 2 diabetes.
Insulin Signaling:
This signaling mechanism is initiated by the binding of insulin to a transmembrane tyrosine kinase receptor. The insulin receptor (IR) autophosphorylates tyrosine residues located in the intracellular portion of the receptor. Rapid phosphorylation of tyrosine residues of insulin receptor substrate 1 through 4 (IRS-1 through IRS-4) occurs quickly after the initial phosphorylation. The IRS residues use a number of pathways, amongst the most prominent is the PI3K/Akt/mTOR pathway2. IRS are widely distributed in the brain and enriched in the hypothalamus, hippocampus, cerebral cortex, cerebellum, and olfactory bulb2.
Insulin Defect in AD:
Postmortem studies of AD brains have revealed defective insulin signaling2. Insulin resistance can be caused by b-amyloid oligomers (AbOs), proximal toxins that conglomerate in AD brains2. AbOs cause rapid insulin insensitivity when exposed to hippocampal neurons2. Different molecular mechanisms are associated with this defective insulin signaling that connect AD and diabetes.
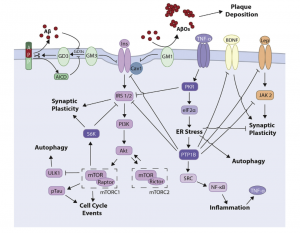
Fig 1. Representation of the insulin signaling pathway and associated mechanisms in AD and T2D.
- Inflammation:
Insulin resistance in diabetes can be attributed to mild, sustained inflammation of peripheral tissue2. Fat accumulation in the adipose tissue causes macrophage recruitment and secretion of TNF-a, a proinflammatory cytokine2. Similarly, AD brains are also characterized by sustained chronic inflammatory state. Microglial activation and secretion of proinflammatory cytokines, such as TNF-a, can be instigated by AbOs in vitro, in mice2. Moreover, AbO induced insulin resistance requires the TNF-a receptor (TNFR)2.
- Gangliosides:
Insulin resistance may proceed by another mechanism that includes gangliosides, complex lipids that are located in the gray matter of the human brain. Mounting evidence exhibits that interactions between ganglioside GM3 and IRS-1 could be that mediator of insulin resistance. Inhibition of GM3 ultimately causes the uncoupling of IR from IRS-1 and consequent insulin resistance2. GM1 ganglioside has also been associated with insulin resistance. This sensitivity is caused by a build-up of GM1, due to uncertain mechanisms2. In AD, the interaction between AbOs and the ganglioside GM1 has been object to excessive investigation. GM1 is a binding site for AbOs on the neuronal membrane. This action promotes aggregation of the peptide into the toxic amyloid structures2. AbOs aggregate forming the plaques that are common with AD pathogenesis. GM3 is also a culprit in AD pathogenesis. This ganglioside may accumulate in the membrane due to AbOs triggering defective metabolism of GM32. As aforementioned, a build-up of gangliosides causes insulin resistance; thus AD could ensue.
- mTOR:
In the brain, development is heavily dependent on mTOR and its incorrect activation can lead to neurodegeneration2. mTOR activation has been correlated with IRS-1 inhibitory phosphorylation, which uncouples the interaction of IRS-1 with IR2. Insulin resistance in AD has also been characterized from increased activation of the mTOR pathway and inhibitory phosphorylation of IRS-12. The mTOR pathway is major interest between insulin resistance and AD, as it may impact insulin signaling regulation, autophagy, and subsequently, AbO clearance; all of which are common mechanisms of AD pathogenesis.
- PTP1B:
Recent research demonstrates important roles PTP1B in the CNS. The protein tyrosine phosphatase 1B (PTP1B) is an important factor of insulin signaling2. PTP1B is a positive regulator of microglia-mediated neuroinflammation2. This neuroinflammation is s common event in AD pathogenesis that is implicated by the development of neuronal insulin resistance.
Impact of Alzheimer’s Disease:
These studies suggest a strong association between AD and T2D. Many mechanisms have been explored; however, every mechanism of AD and T2D seems to have one common modulator – insulin resistance. Type 2 diabetes (T2D) nearly doubles the risk of dementia2. As type 2 diabetes prevalence increases throughout the United States, increasing risk of AD increases as well. In my opinion, AD is one of the most devastating diseases known. The disease takes the mind, then it takes the body. AD strips patients of their memories and cuts them off from the world. Families are forced to watch their loved ones with AD forget who they are and the people in their lives’. Hopefully, watching what we consume food wise will decrease the risk of developing AD. Next time, ignore your sweet tooth and put down the cupcake.