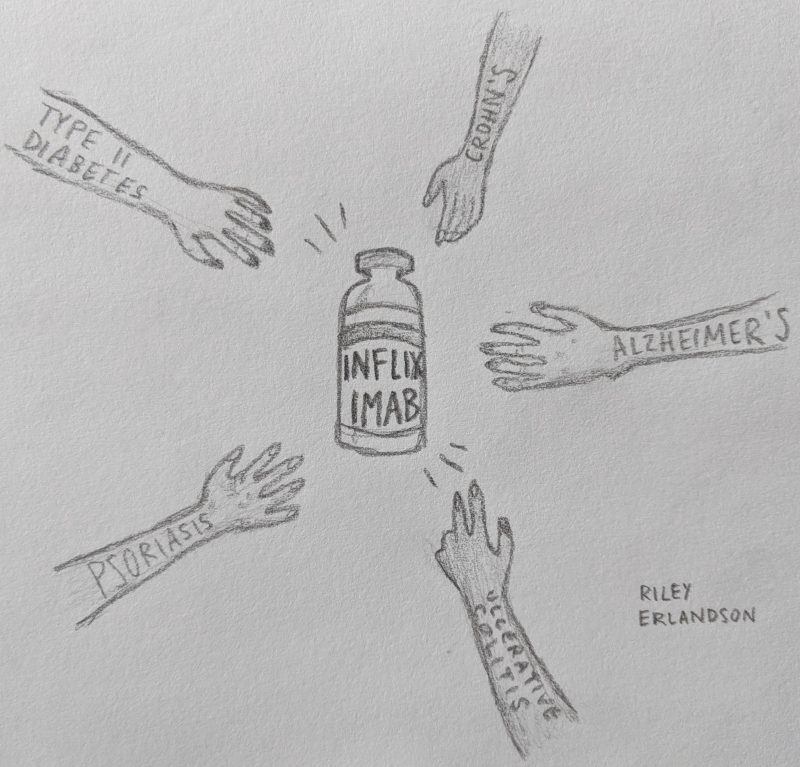
What would you think if I told you the same manmade drug is used to treat Alzheimer’s Disease (AD), Type II Diabetes (T2D), psoriasis, ulcerative colitis, Crohn’s disease, rheumatoid arthritis, and more seemingly unrelated disorders? Are you skeptical that these diseases have enough in common that the same drug is prescribed for them all? Read on to find out how much these disorders have in common on a molecular level and how one drug helps treat them all.
Molecular causes of AD and T2D
First, let’s look at what is happening on the molecular level in AD and T2D. As you may know, T2D is characterized by insulin resistance—an inability to produce or process insulin the way the body is supposed to. This results in T2D disease progression, especially increased blood sugar (hyperglycemia). However, insulin also plays an important role in the brain. It helps protect neurons, the brain’s cells, and is an important player in learning and memory pathways. In this way, insulin resistance has been shown to contribute to AD disease progression as well. The two disorders have a high comorbidity, meaning the often occur together, and a similar molecular cause: inflammation.
 When functioning normally, insulin in the brain binds to insulin receptors located on the neuron’s cell membrane. These receptors, once activated, phosphorylate proteins called IRS1 and IRS2, which means that phosphate molecules are put on the proteins. This in turn activates IRS1 and IRS2 which go off to cause more activation and signaling cascades resulting in insulin’s aforementioned activities in the brain, like facilitating learning and memory.
When functioning normally, insulin in the brain binds to insulin receptors located on the neuron’s cell membrane. These receptors, once activated, phosphorylate proteins called IRS1 and IRS2, which means that phosphate molecules are put on the proteins. This in turn activates IRS1 and IRS2 which go off to cause more activation and signaling cascades resulting in insulin’s aforementioned activities in the brain, like facilitating learning and memory.
However, in AD, this signaling chain is interrupted. Cells called macrophages, which play an important role in the immune system and in causing programmed cell death, become active. These macrophages secrete proinflammatory cytokines, which are chemicals that cause inflammation. One important proinflammatory cytokine in AD is called TNF-α. High concentrations of TNF-α cause significant inflammation, which interrupts the insulin signaling pathway by preventing insulin receptors from initiating IRS1 and IRS2 phosphorylation. This is how insulin resistance happens in the brain. TNF-α prevents the whole chain of signaling events meant to occur after insulin is released, keeping those important functions like learning and memory from occurring as they should.
Where Infliximab comes in
The drug Infliximab counters this inflammatory insulin resistance pathway. Infliximab is a monoclonal antibody, meaning a manmade (not naturally occurring) drug that acts as an antibody by binding to and neutralizing an antigen, the harmful substance targeted. The antigen targeted by Infliximab is TNF-α. The drug binds to TNF-α and neutralizes it so it cannot cause inflammation and in turn insulin resistance.
Infliximab is highly specific to TNF-α, so it doesn’t bind to any other TNFs (other like TNF-β exist), which is useful because they have their own functions in the cell beyond inflammation that could be harmful if interrupted.
Because it interrupts this inflammatory pathway that contributes to AD and T2D disease pathology, Infliximab has been used experimentally to treat the disorders with relative success. The drug is already prescribed to treat a wide variety of other disorders, as mentioned above: Crohn’s disease, psoriasis, ulcerative colitis, etc. Its mechanism of action works to treat each disorder because inflammation is a key component of them all. It’s clear that mitigating inflammation in the brain and body can be useful in treating or even preventing a wide array of serious disorders.
So, should I just start taking it now?
 Definitely not! First of all, Infliximab is not a preventative drug meant to keep you from developing AD or T2D. This drug is prescribed in severe cases of the aforementioned disorders for which other treatments have not been successful. Infliximab can have some pretty serious side effects including causing infections, leukemia, and demyelinating central nervous system disorders. It’s also administered by shot every 6-8 weeks and costs about $900 a dose—not something you should go for if it hasn’t been prescribed by a doctor.
Definitely not! First of all, Infliximab is not a preventative drug meant to keep you from developing AD or T2D. This drug is prescribed in severe cases of the aforementioned disorders for which other treatments have not been successful. Infliximab can have some pretty serious side effects including causing infections, leukemia, and demyelinating central nervous system disorders. It’s also administered by shot every 6-8 weeks and costs about $900 a dose—not something you should go for if it hasn’t been prescribed by a doctor.
If you’re looking for a preventative measure to counteract inflammation in hopes of delaying or preventing disease onset, you should take a look at a few other “Cobbers on the Brain” posts to see how things like antioxidants and CBD can work to fight inflammation and potentially protect against developing AD, T2D, and other inflammation disorders.