Let us take a quick look at what the endocannabinoid system actually is. There are two major endocannabinoids in the body, specifically the brain: Anandamide (AEA) and 2-Aracidonoglycerol (2-AG). These molecules are considered to be lipophilic signaling molecule which can be released into the CNS via intracellular Ca2+ levels increasing or activation of metabotropic receptors. When endocannabinoid molecules are released into the post synapse of the neuron, it will activate the CB1 receptor as well as other GPCRs. The CB1 receptor is considered to target motor activity, appetite, immune cells, short-term memory, and pain perception. However, there is another receptor, the CB2 receptor, that is activated in a similar manner. This receptor is more associated with the peripheral nervous system and the immune system. The CB2 receptor targets areas, such as the kidneys, liver, eyes, gut, skin, reproductive system, and the cardiovascular system. Once these receptors have activated their appropriate cascade, then the endocannabinoid may be broken down via hydrolytic bond cleavage or by enzyme breakdown. Enzymes that help break down AEA and 2-AG include lipoxygenases and cytochrome P450.
Looking from the above mechanism, it seems that endocannabinoids play a relatively large role in everyday functioning. In terms of pain perception, activation of the endocannabinoid system helps to inhibit the pain cascade and can mitigate pain symptoms. Other studies have begun looking at using the activation of CB1 to increase appetite and combat certain eating disorders. Activation of the endocannabinoid system can sometimes mean using an exogenous source, such as ingesting marijuana in some manner. CBD is a naturally producing chemical in the body, and it is the influx that begins to show therapeutic treatment. However, the efficacy and ethicality of medical uses of marijuana is a conversation for another blog post.
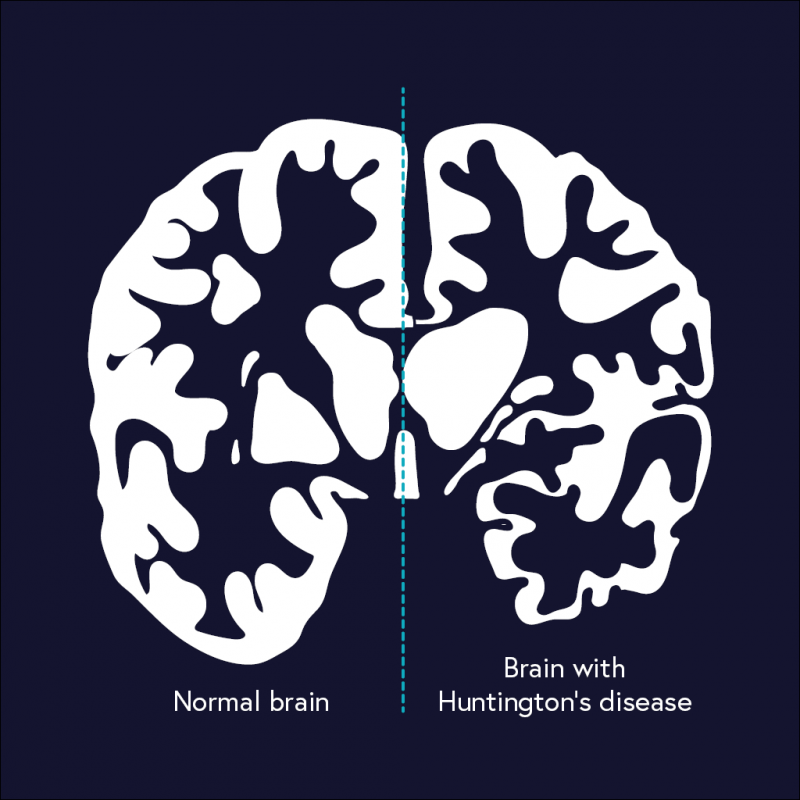
One disease however seems to defy the beneficial therapeutics of medical intervention of the endocannabinoid system. Huntington’s Disease (HD) is a genetic mutation of the IT-15 gene causing an abnormal number of nucleotide repeats. These nucleotide repeats, often called polyglutamine (PolyQ) stretches can promote cell death and aggregation. When these PolyQ stretches continue to grow in size, the more likely the stretch to form a beta sheet. When a beta sheet is formed, chaperone proteins label it as misfolded and cause aggregation, which can be cytotoxic in nature. Early symptoms of HD include stumbling, difficulty concentrating, depression, and memory lapses. Further progression of the disease can lead to severe motor dysfunction, such as fidgety movements, trouble breathing, difficulty speaking, and/or trouble swallowing.
There is little to no possible therapeutics for HD at the moment, slightly due to the unknown diversity in size and cytotoxicity levels of the aggregates. One study by Xi et al. (2016) did examine activating CB1 receptors to alleviate symptoms. They noticed a decrease in HD-induced cell death, but the total number of aggregates increased significantly. This was also seen for increasing cAMP. However, another study by Xie et al. (2010) showed that forced influx of BDNF in the striatum has been shown to prevent cell death and prevent some of the motor dysfunction symptoms. It was also shown to reverse decreased dendritic spine density and decrease abnormal spine morphology commonly seen in HD.
In conclusion, maybe Huntington’s can not be treated via the endocannabinoid system, but there are promising outcomes if CB1 receptors are activated. If the nature of the HD aggregates could be made soluble, then CB1 activation could be used as a therapeutic intervention in conjunction with other therapeutics.
Photo Sourced From: https://www.science.org.au/curious/people-medicine/huntingtons-disease