An Overview of Alzheimer’s Disease:
Alzheimer’s Disease (AD) is a neurodegenerative disease characterized by progressively worsening executive cognitive functioning, including memory impairment and overall cognitive deficits. The symptoms start small and may not even be noticed but for the following years, the disease will become much more apparent. Hyperphosphorylation of the tau protein causes the production of neurofibrillary tangles and amyloid-beta plaques, which trigger neuroinflammatory pathways, eventually leading to neurodegeneration/apoptosis, decreasing brain size. 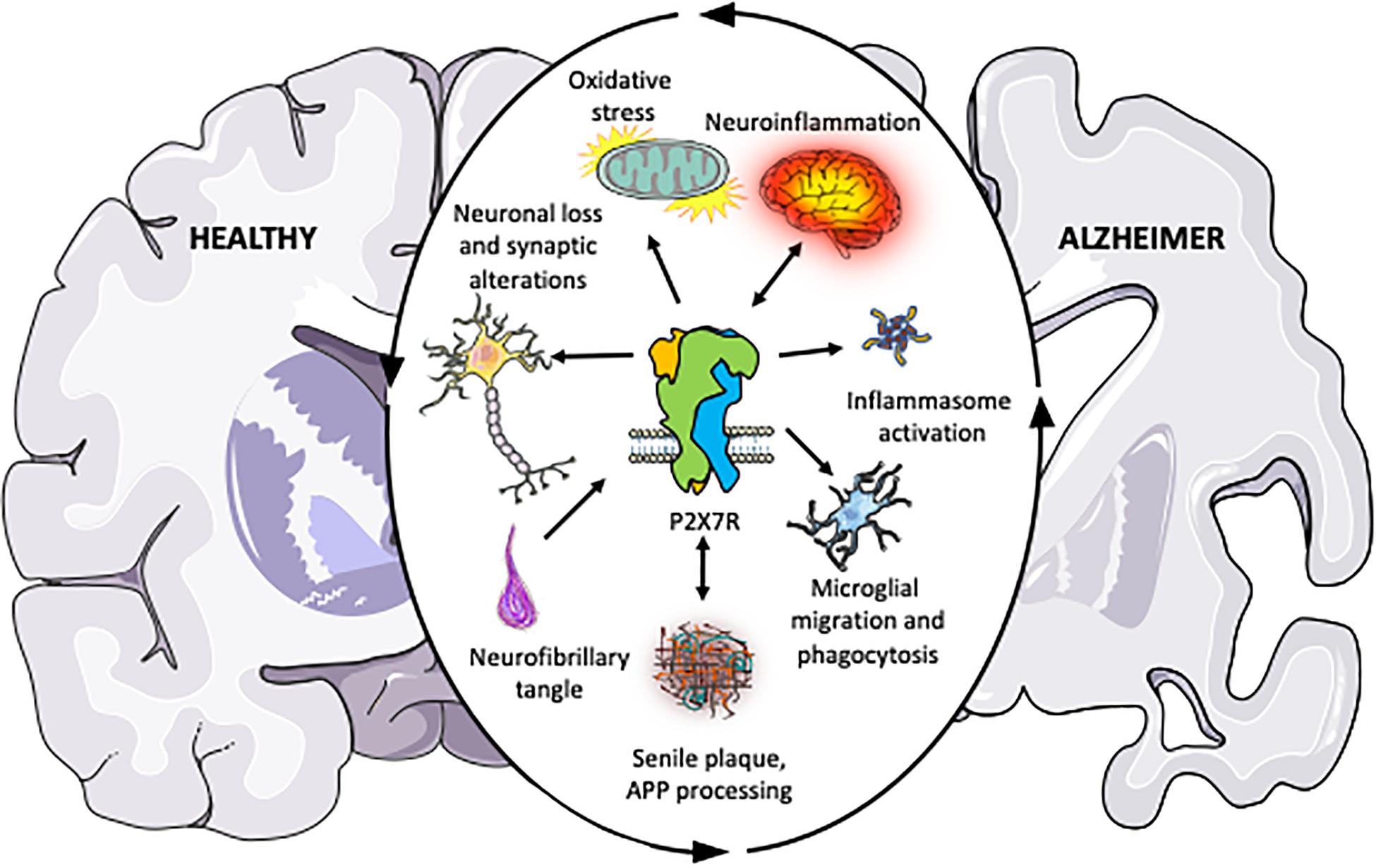
The Caspase Family:
The caspase family includes caspase 1-12 and are all protease enzymes that play significant roles in neuroinflammatory and pro-apoptotic processes. As proteases, these enzymes (caspase 3, 6, and 7) break down proteins into peptide chains, then further into individual amino acids.
The inflammatory caspases (1, 4, 5, 8, and 11) initiate the inflammatory response after a pro-inflammatory cytokine binds to the corresponding receptor. Different caspases communicate with each other to transduce the cytokine’s signal to the cell nucleus, where pro-inflammatory gene transcription is upregulated, leading to higher degrees of cell inflammation. In terms of AD, inflammatory caspases can aggravate the neurofibrillary tangles and amyloid plaques, which can trigger neurodegenerative processes, eventually leading to apoptosis. 
Crystal structure of Caspase-3 (3DEI), the caspase gaining most attention as a possible therapeutic target
The pro-apoptotic and neurodegenerative caspases (2, 8, 9, and 10) are responsible for initiating the apoptotic pathway, leading to the breakdown of various proteins inside the cell into peptides and amino acids, which can lead to the neuron or cell becoming nonfunctional, which triggers programmed cell death pathways. This explains why post-mortem analyses of AD brains are significantly smaller in size than non-AD brains.
The figure below illustrates this idea of how NFT/AB plaques, neuroinflammation, and neurodegeneration are all related through the caspase proteases. https://pubmed.ncbi.nlm.nih.gov/31111399/
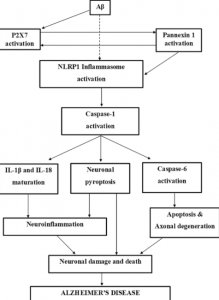
Apoptosis and Neurodegeneration:
It is important to note that caspase-mediated neuroinflammatory and neurodegenerative pathways are not themselves impaired in AD progression. Caspases do not like neurofibrillary tangles or amyloid plaques. Their interaction stimulates cytokine release and the pathway that follows responds the way it is supposed to. These proteases are behaving exactly the way they are meant to, but the dysfunction of the upstream pathways is constantly triggering these events.
Can we Treat AD with Caspase Inhibitors?
The short answer is yes, but it may not be a very effective therapeutic aspect in terms of “curing” Alzheimer’s. The formation of neurofibrillary tangles and amyloid-beta plaques is a required prerequisite for hyperstimulating pro-inflammatory cytokine release and thus neurodegeneration and apoptosis. These steps are consequences far downstream from severe kinase pathway dysfunction and developing successful caspase-targeted drugs would not do anything to stop or retard the progression of the disease.
Caspases are at the end of the signal transduction pathway. The progression of AD is not a simple, one-road track. This disease is caused by severe dysfunction of nearly every protein involved in an already complex signal transduction pathway. The caspases are far downstream from these proteins and their actions are a result of chaotic impairment of upstream functioning. Developing a caspase inhibitor could marginally increase the cell lifespan, but in terms of AD pathogenesis, it would do nothing to prevent the disease from progressing. This paper goes further into the neurochemistry of caspase-mediated apoptosis in AD and explains caspase-targeted drug outlook more in-depth. https://pubmed.ncbi.nlm.nih.gov/11556539/
Summary
Caspases are largely responsible for inflammation, neurodegeneration, and apoptosis in Alzheimer’s Disease. While their specific pathways are not dysfunctional themselves, their activity is a result of dysfunction throughout upstream pathways that lead to tau hyperphosphorylation. As proteases that are towards the end of the AD-progressing pathway, using them as drug targets could prolong cell life and may increase cell lifespan at best, but the progression of the disease is much more complicated.