Addiction


Artstract. Art found online that I believe depicts the detrimental impact psychostimulant drug use has on our brains.
Psychostimulant Use Disorder
Psychostimulant Use Disorder (PUD) causes changes in the brain’s reward circuit and glutamatergic neurotransmission. Because amphetamine-like psychostimulants have a similar structure (phenylalkylamines) to the endogenous catecholamine neurotransmitters (norepinephrine, epinephrine, and dopamine), psychostimulants are theorized to alter catecholamine neurotransmission. These changes lead to dopamine-driven reinforcement and drug-seeking behaviors (1).
Nothing in PUD makes sense, except in the light of altered brain neuroplasticity.
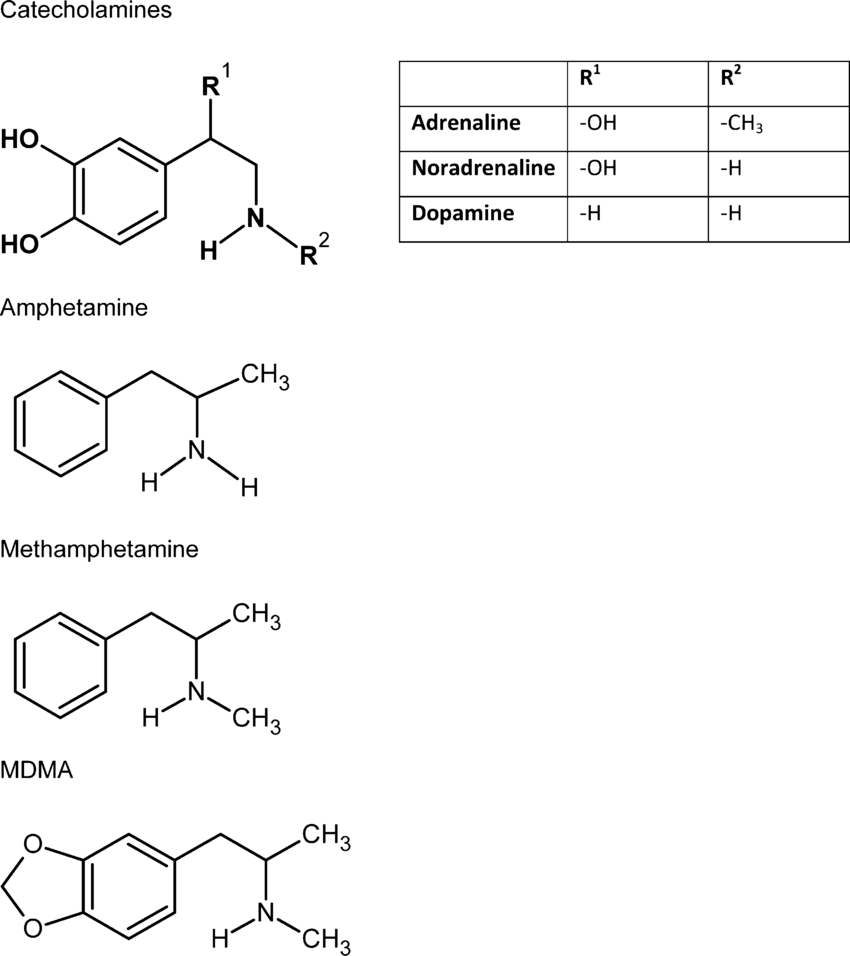 Figure 1. Psychostimulant structure compared to catecholamine neurotransmitters (2).
Figure 1. Psychostimulant structure compared to catecholamine neurotransmitters (2).
Psychostimulants
Psychostimulants are drugs that increase (“stimulate”) the CNS. They bind to extracellular catecholamine receptors (i.e. DA) to promote the release of neurotransmitters (NTs). They can also suppress monoamine NT reuptake and oxidase metabolism, which leads to elevated levels of NTs in the synapse.
Glutamate
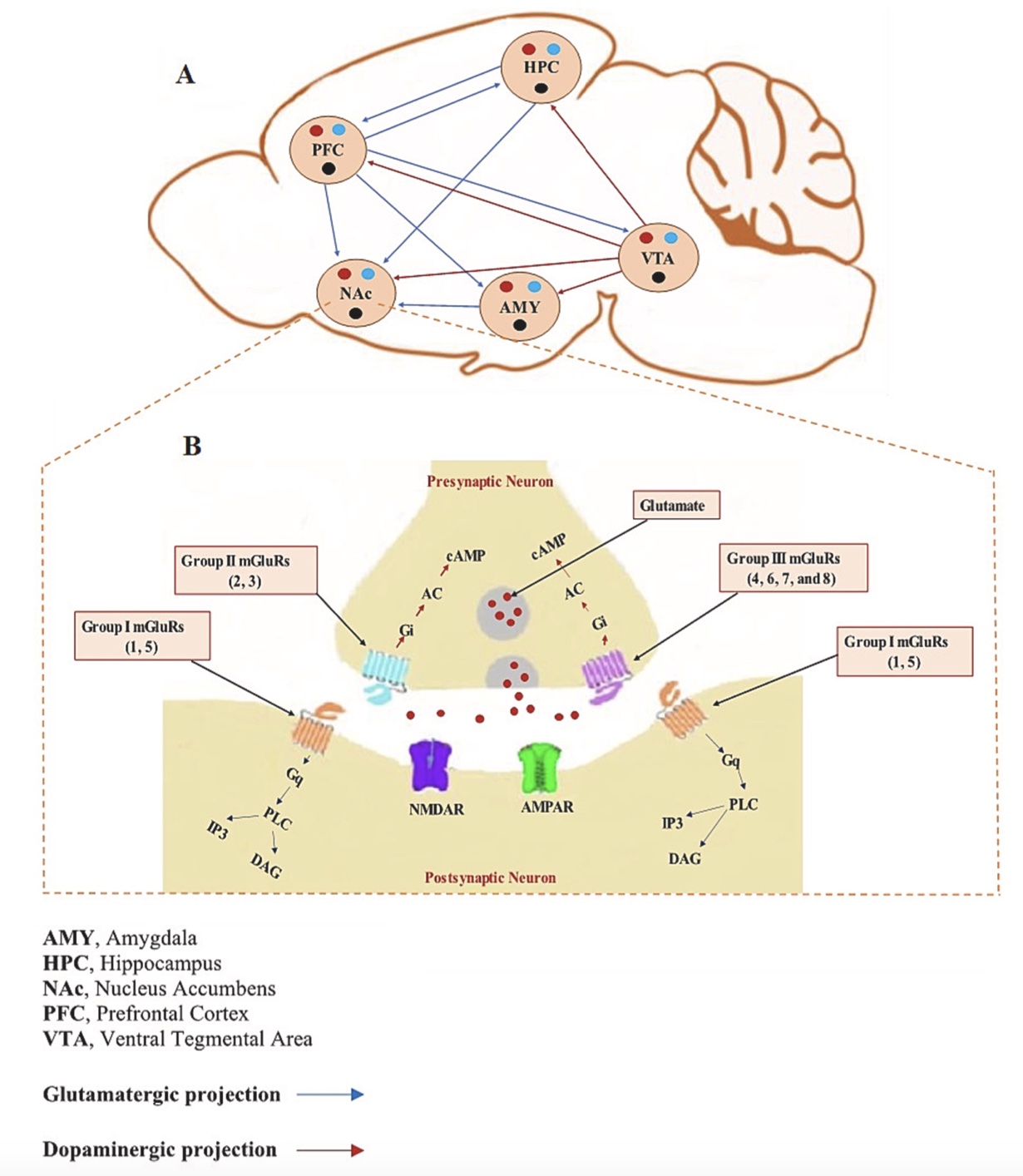
Glutamate is the primary excitatory neurotransmitter in the central nervous system. Modulating glutamate neurotransmission has emerged as a promising approach for treating cognitive deficits associated with substance use disorders (SUDs), including PUD. When glutamate is released from presynaptic neurons, it diffuses through the synapse and binds to various glutamate receptors including ionotropic (such as NMDA and AMPA) receptors that are fast-acting and crucial for brain plasticity as well as metabotropic receptors. Metabotropic glutamate receptors are categorized into three groups:
Group 1
- mGluR1 & mGluR5
- postsynaptic
- Gq-coupled.
Group 2
- mGluR2 & mGluR3
- presynaptic
- Gi-coupled.
Group 3
- mGluR4 & mGluR6 & mGluR7 & mGluR8
- presynaptic
- Gi-coupled.
Figure 2. Distribution of mGluRs in the Brain’s Reward Circuit (1).
Neurocircuitry of SUDs: The Brain’s Reward Circuit
The reward circuit involves intricate interactions between dopaminergic projections from the ventral tegmental area (VTA), glutamatergic and GABAergic neurotransmission, and brain regions like the medial prefrontal cortex (mPFC) and nucleus accumbens (NAc).
Dopamine activity in the brain is modulated by the VTA brain region. In reward processing and drug-seeking behaviors, glutamatergic neurons from the mPFC stimulate the VTA’s dopaminergic neurons to release dopamine in the NAc (1).
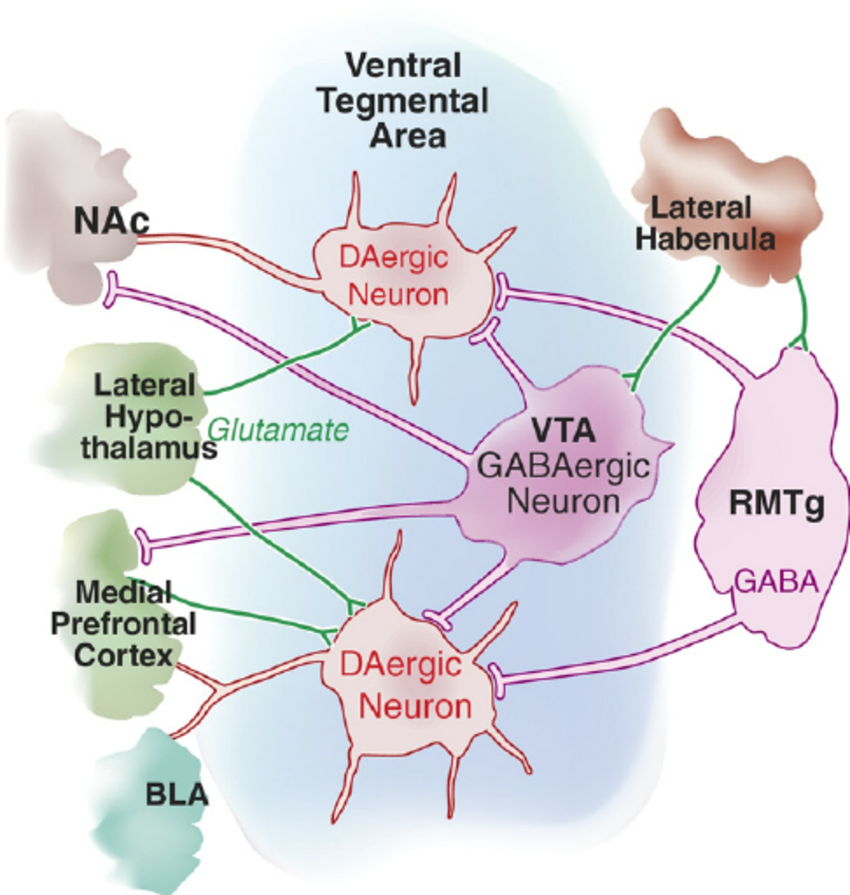
Figure 3. VTA plays an important role in the brain’s reward circuit (3).
Neuroplasticity and Memory Formation in PUD:
Alterations in synaptic strength, dendritic morphology, and receptor expressions are found in neurons associated with the brain’s reward circuits. Long-term potentiation (LTP) and long-term depression (LTD) in glutamate synapses, particularly in the hippocampus and mesocorticolimbic pathways play a crucial role in the altered neuroplasticity that comes from drug abuse.
Group 1 mGluRs have been found to affect synaptic plasticity in reward circuit brain regions in PUD cases. They modulate short- and long-term synaptic plasticity, including LTD, through intracellular signaling pathways involving phospholipase C (PLC) that increase learning, memory, and LTP. mGluR1 is thought to be involved in the acquisition process while mGluR5 has a role in retaining and maintaining spatial memories. Because group 1 mGluRs are located on the postsynaptic neuron, antagonist drug treatments have been found to reduce drug-seeking behaviors by blocking the reward signal from being passed on (1).

Figure 4. Antagonist drug treatments have been found to reduce addiction when targeting Group 1 receptors because they inhibit mGluRs on the postsynaptic cell.
Group 2 mGluRs negatively regulate glutamate release and are necessary for inducing LTD in the reward circuit. Because group 2 mGluRs are located on the presynaptic neuron, agonist drug treatments have been found to reduce drug-seeking behaviors by stimulating auto receptors to decrease the number of neurotransmitters released in the synapse (1). Group 3 mGluR’s role in PUD synaptic plasticity needs to be studied further, particularly mGluR7 and 8 in regions like the NAc, VP (striatum), and the hypothalamus.

Figure 5. Agonist drug treatments have been found to reduce addiction when targeting Group 2 receptors because they stimulate the mGluR auto receptors that self-regulate by reducing NT release.
Questions to Think About…
- Does the altered brain chemistry from addiction ever go back to the way it was?
- Can we unlearn memory/ the motivated driver of addiction?