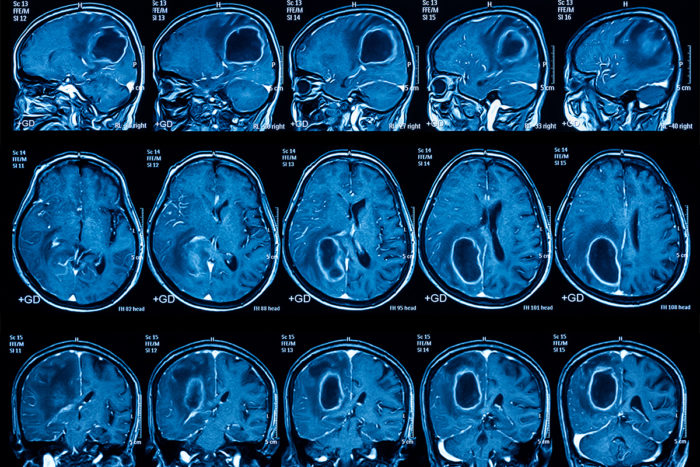
Glioblastomas
Glioblastomas (GBM) are the most common and deadly malignant brain cancer. These brain tumors are aggressive, with their complex genetic makeups and relentless growth patterns. Because of its complexity GBMs are hard to treat, current treatments rely on surgery, chemotherapy, and a single drug – TMZ. GBMs can manifest as primary tumors, emerging rapidly without warning, or as secondary tumors evolving from lower-grade gliomas over several years.
There are four subtypes of GBMs, each with its own molecular fingerprints. The classical subtype is characterized by amplified epidermal growth factor receptor gene (EGFR) and non-mutated TP53 proteins. In contrast, the mesenchymal subtype has common mutations in neurofibromin 1, PTEN, and TP53. These genes are involved in MAPK and PI3K signaling pathways and contribute to tumor cells’ invasiveness and drug resistance. Other subtypes show the heterogeneity challenges of GBM, with pro-neural subtypes classifying younger patients who have multiple genetic mutations and neural subtypes with the worst survival rate and no common genetic pattern.
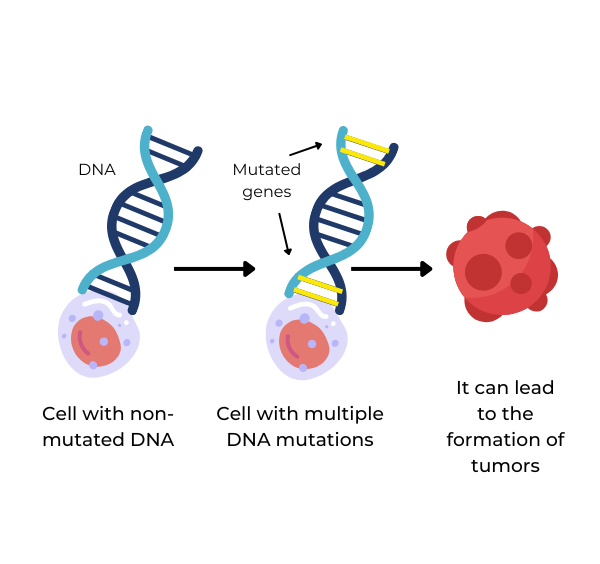
Figure 1. Gene mutations can lead to the formation and invasiveness of tumor cells (3).
A recent paper looking into the underlying mechanisms of GBMs highlighted the potential to target convergence points and crosstalk of signaling pathways involved in cancer pathogenesis.
Signaling Pathways that Promote GBM Carcinogenesis
Hyperactivation of the pathways seen below in Figure 2, often stemming from gene mutations that cause EGFR amplifications or PTEN inactivation, increases tumor growth and GBM progression.
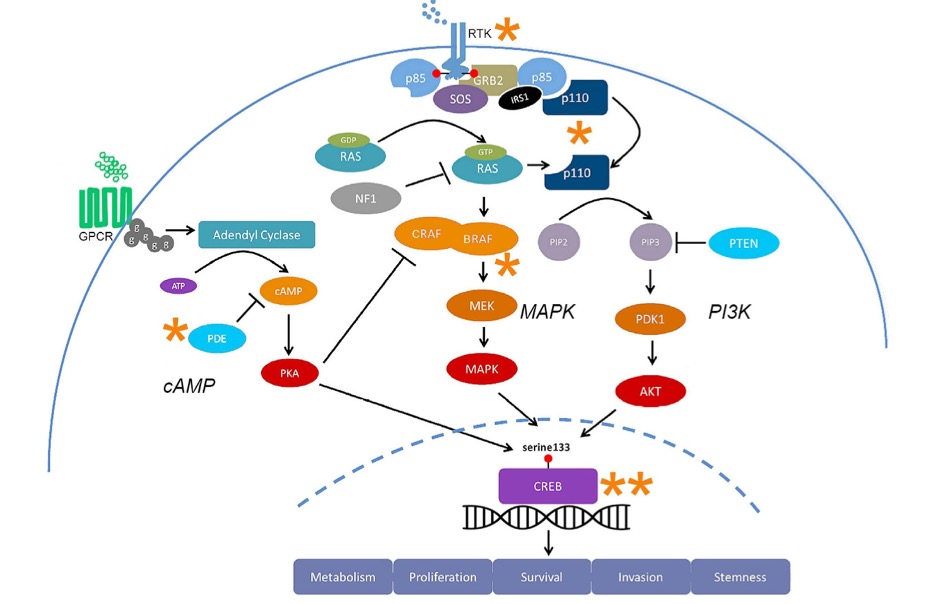
Figure 2. Multiple signaling pathways including MAPK, PI3K, and cAMP contribute to cancer pathogenesis (1).
MAPK Pathway
This pathway regulates cell proliferation (cell survival) and metastasis (how cancer spreads). In cancer, the MAPK pathway is hyperactive because of the mutation-driven higher levels of EGFR growth factors. EGFR activates the MAPK pathway that promotes proto-oncogene transcription factors like Elk1 and CREB to allow tumor cells to multiply. The gene NF1 negatively regulates the MAPK pathway by converting GTP to GDP, inhibiting Ras. This genetic component is seen in 14% of mesenchymal GBM cases, as NF1 is depleted or mutationally inactivated, leading to hyperactive MAPK pathways.
PI3K Pathway
This pathway regulates cell growth and when dysregulated promotes tumor cell invasion. In GBM, PI3K pathways are hyperactive because of genetic mutations that lead to increased levels of EGFR, PIK3CA (the gene that encodes the p110 catalytic subunit of PI3K), and inactivated PTEN genes. Growth factors and Ras activate the PI3K pathway activates the mTOR pathway and transcription factors that promote cell growth. The PTEN gene negatively regulates the PI3K pathway by converting PIP3 to PIP2, leading to the inhibition of Akt, an important role in moderating normal cell growth levels. Disrupted PI3K pathways increase MMP levels, which lead to degraded extracellular matrices and contribute to GBM tumor cell invasiveness.
Tumor Cells and Drug Resistance
GBMs are very resistant to drug treatment because of the multiple signaling pathways that promote tumor growth—MAPK and PI3K pathways. Drug resistance is common in GBMs because targeting one pathway leads to the tumor cells using another pathway to continue its proliferation. This ability to resist is why current drugs that target convergence points of the multiple pathways involved are being researched (the asterisks in Figure 2 above are the possible convergence points).
Potential Drug Targets Focused on Convergence Points Between Pathways
The intricate crosstalk between MAPK and PI3K pathways further complicates therapeutic interventions. While both pathways can be activated by common receptors and Ras proteins, they also exhibit cross-inhibition and cross-activation mechanisms involving their main molecules (GAB1, ERK, TSC1/2, mTORC1, and Akt). Targeted inhibitors against PI3K, MAPK, EGFR, and CREB signaling have shown promise in preclinical studies, yet the challenge of adaptive pathway resistance to single drug targets has highlighted the potential of a combination of drug targets as a future treatment strategy.
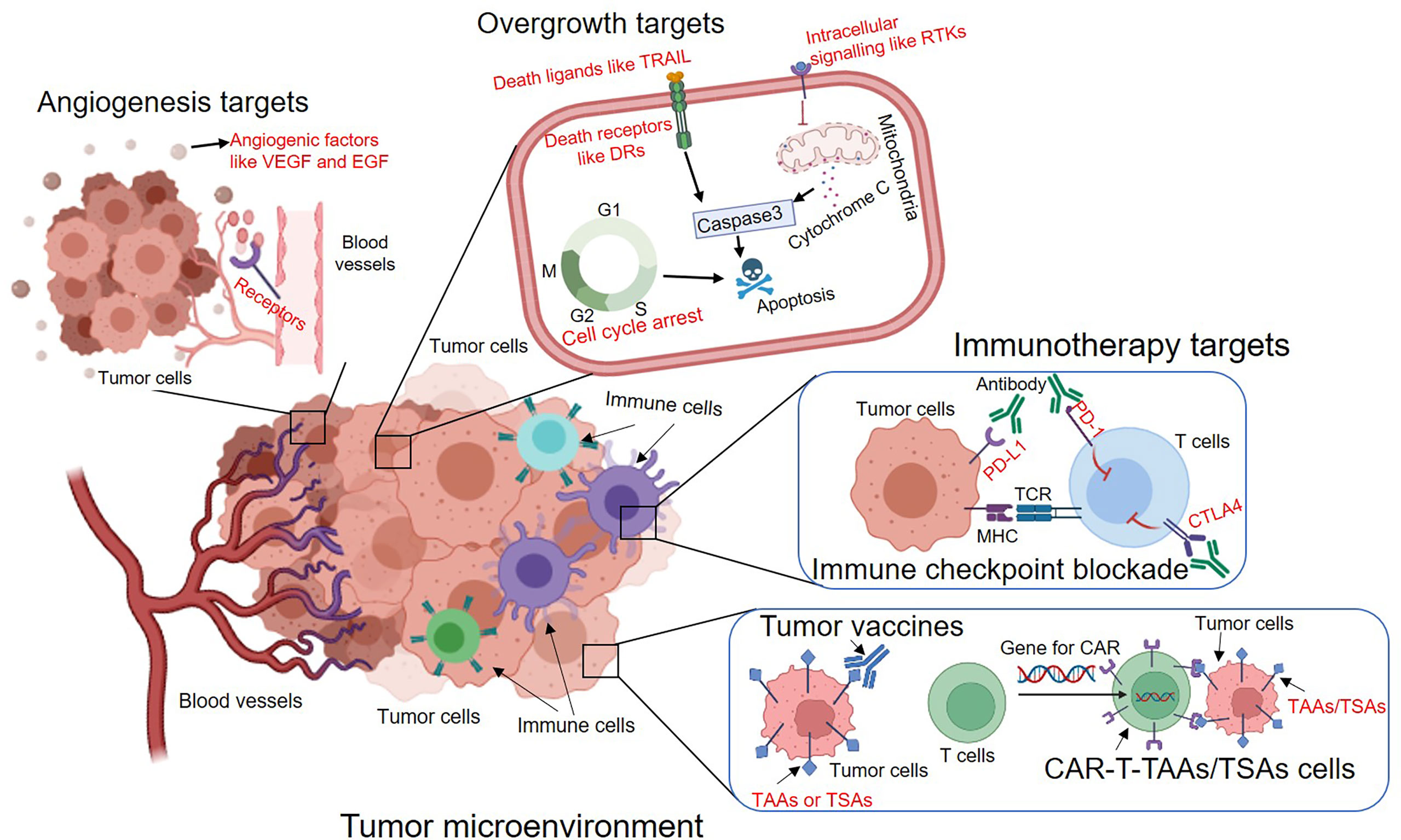
Figure 3. Tumor microenvironments are important to consider when considering what to target in drug treatments (4).
Nothing in Glioblastomas makes sense, except in the light of mutationally amplified EGFR genes causing hyperactivation of both the MAPK and PI3K pathways, leading to GBM carcinogenesis.