Picture 1: Glioblastoma pictured in the brain.
Glioblastoma (GBM) is an aggressive and invariable lethal form of cancerous brain tumor, and it’s among the types of cancer with the worst survival rates. This type of cancer is extremely invasive, and spreads rapidly to neighbouring structures as well as extending into healthy brain tissue[1]. There are two main types of GMB, which is primary and secondary. Primary GBM develop without evidence of any pre-existing symptoms or tumor, while secondary arises from a lower grade tumor. Additionally, there is various molecular subtypes of GBM, which are classical, mesenchymal, proneural and neural GBM[2].
The current standard treatment for GBM is surgery, radiotherapy, and a singular drug called temozolomide. The limited therapy is associated with GSM being relatively resistant to therapy, and due to the drug needing to cross the Blood Brain Barrier (BBB), additionally exhibiting robust radio-resistance[3].
Three cellular transduction pathways have been found to play a role for GBM, these are the MAPK, PI3K, and cAMP pathways:
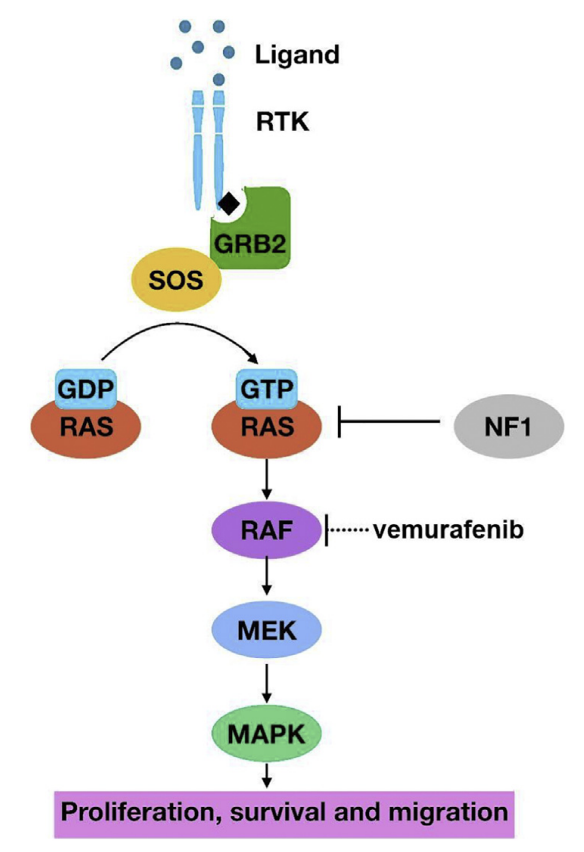
Figure 1: The MAPK pathway, from the activation by ligands to its downstream effects[4].
The MAPK pathway was found to be frequently altered in GBM, and high levels of phosphorylated (or activated) MAPK being linked to poor survival among patients with GBM. Multiple components of this pathway is transformed in cancer leading to the hyperactivation[5].
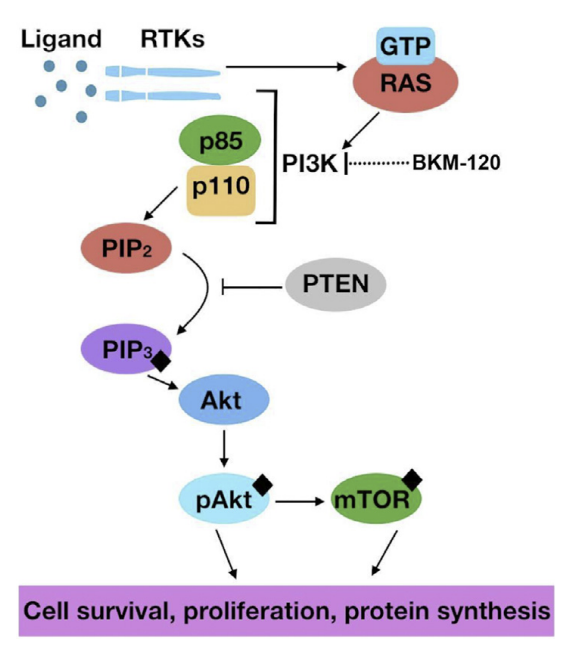
Figure 2: The PI3K pathway, visualized from ligand binding to promoting various factors such as cell survival[6].
The PI3K regulator multiple cellular functions, and is frequently disturbed in cancer whereas components may be mutated or amplified. In GBM, mutations or amplicitations can be found of the EGFR, and activated in PIK3CA which is the gene that encodes p110 within the PI3K pathway, and inactivating of PTEN (a tumor suppressor)[7].
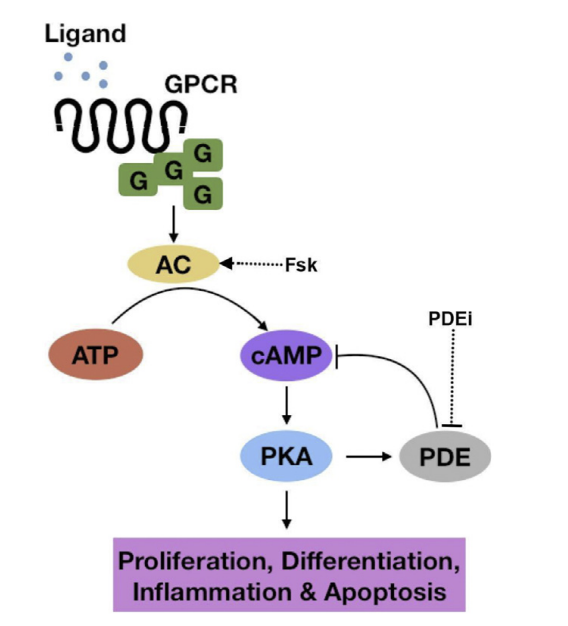
Figure 3: The cAMP pathway, from ligand bind to downstream effects[8].
The cAMP pathway had been less prominent, but tend to be lower in tumor cells. However, it has been shown that the elevation of cAMP levels inhibit growth, increase differentiation as well as promote apoptosis in GBM cells[9].
With the limited amount of treatment currently offered for GBM, opens the discussion for ideas on new therapies or areas of target for treatment. Targeting the different signaling pathways shown to play a role in GBM can be beneficial for treating the tumors. A review from Cellular Signaling brought up a couple of possible treatments, in which are inhibitors and activators that have shown increased apoptosis in cancer cells lines. Some of the various ones that target the three signaling pathways, are a PI3K inhibitor called Buparlisib, a MAPK inhibitor, Vemurafenib, as for cAMP a AC activator called forskolin and a PDE inhibitor, isobutylmethylxanthine[10]. It has however been found that targeting individual pathways is not always that effective. This is because of pathway redundancy, and the ability tumors have to adjust their signaling to other pathways in order to maintain growth and their function. Therefore, targeting areas where the pathways coverage may be effective, which can be cAMP response element-binding protein (CREB). CREB is a transcription factor that crosstalk between these three signaling pathways. The transcription factor is important in a number of downstream functions, which include neurogenesis and cancer. CREB is important for normal brain development. It was found that in mouse brains the deletion of CREB lead to neurodegeneration during development[11].
So, how can CREB be used as treatment for GBM? There has evidence suggesting that CREB is important for regulating tumor initiation, its progression and metastasis, and based on this it has been demonstrated therapeutic potential in possible inhibitors of gene transcription mediated by CREB[12]. The current approaches are termed “CREB inhibitors” and “CREB-related pathways inhibitors”, which can be shown in Figure 4, separated by direct inhibitors in the nucleus, and pathway inhibitors outside of the nucleus[13].
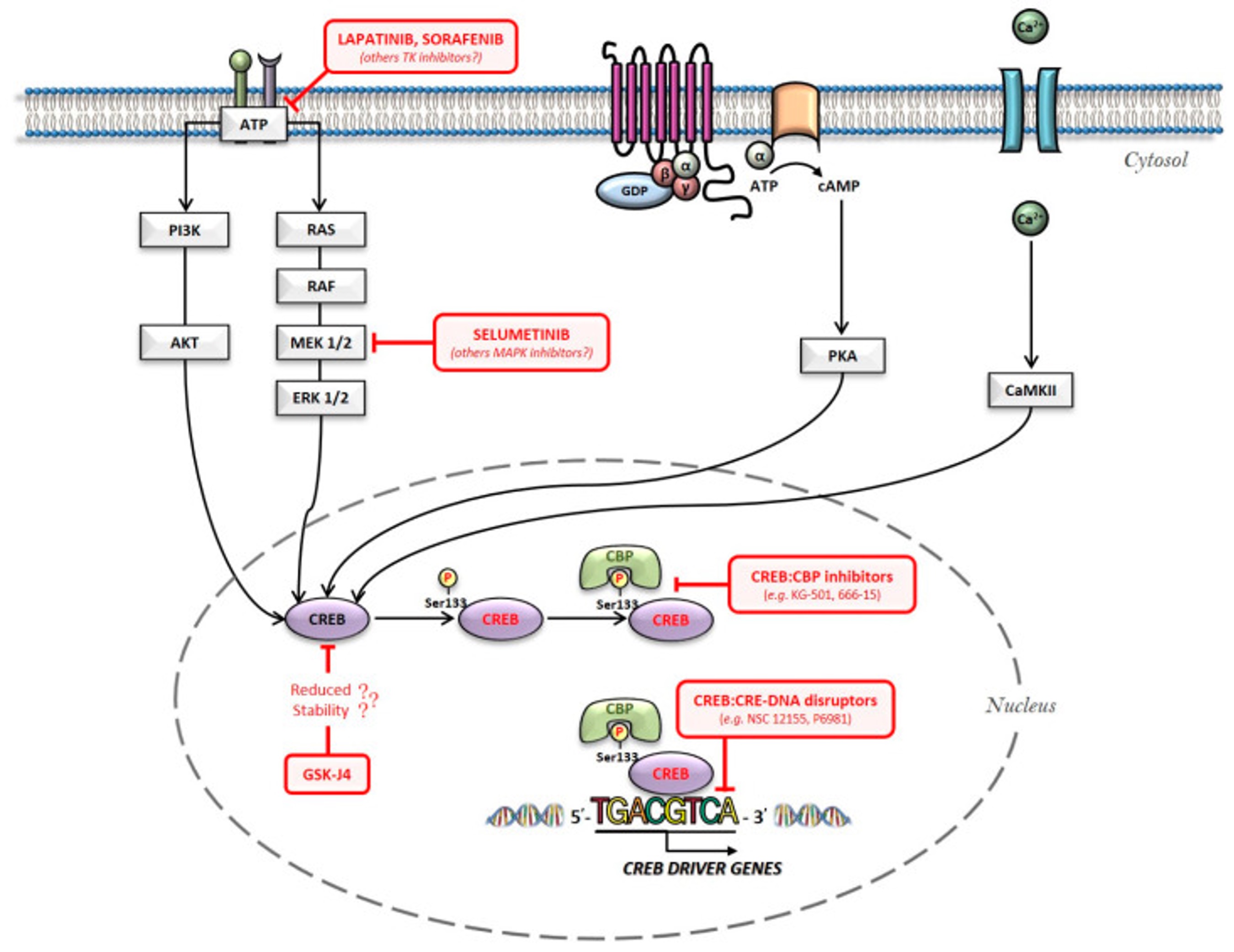
Figure 4: An overview of the main intracellular pathways involved in CREB activation: the red arrows being possible pharmacological hubs for its inhibition[14].
Bibliography
[1] Fung, N.H., Grima, C. A., Widodo, S. S., Kaye, A. H., Whitehead, C. A., Stylli, S. S., & Mantamadiotis, T. (2019) Understanding and expliting cell signalling covergence nodes and pathway cross-talk in malignangt brain cancer. Cellular signalling, 57,2-9. https://doi.org/10.1016/j.cellsig.2019.01.011
[2] Ibid.
[3] Ibid.
[4] Ibid.
[5] Ibid.
[6] Ibid.
[7] Ibid.
[8] Ibid.
[9] Ibid.
[10] Ibid.
[11] Ibid.
[12] Xiao, X., Li, B. X., Mitton, B., Ikeda, A., & Sakamoto, K. M. (2010). Targeting CREB for cancer therapy: friend or foe. Current cancer drug targets, 10(4), 384–391. https://doi.org/10.2174/156800910791208535
[13] Sapio, L., Salzillo, A., Ragone, A., Illiano, M., Spina, A., & Naviglio, S. (2020). Targeting CREB in Cancer Therapy: A Key Candidate or One of Many? An Update. Cancers, 12(11), 3166. https://doi.org/10.3390/cancers12113166
[14] Ibid.