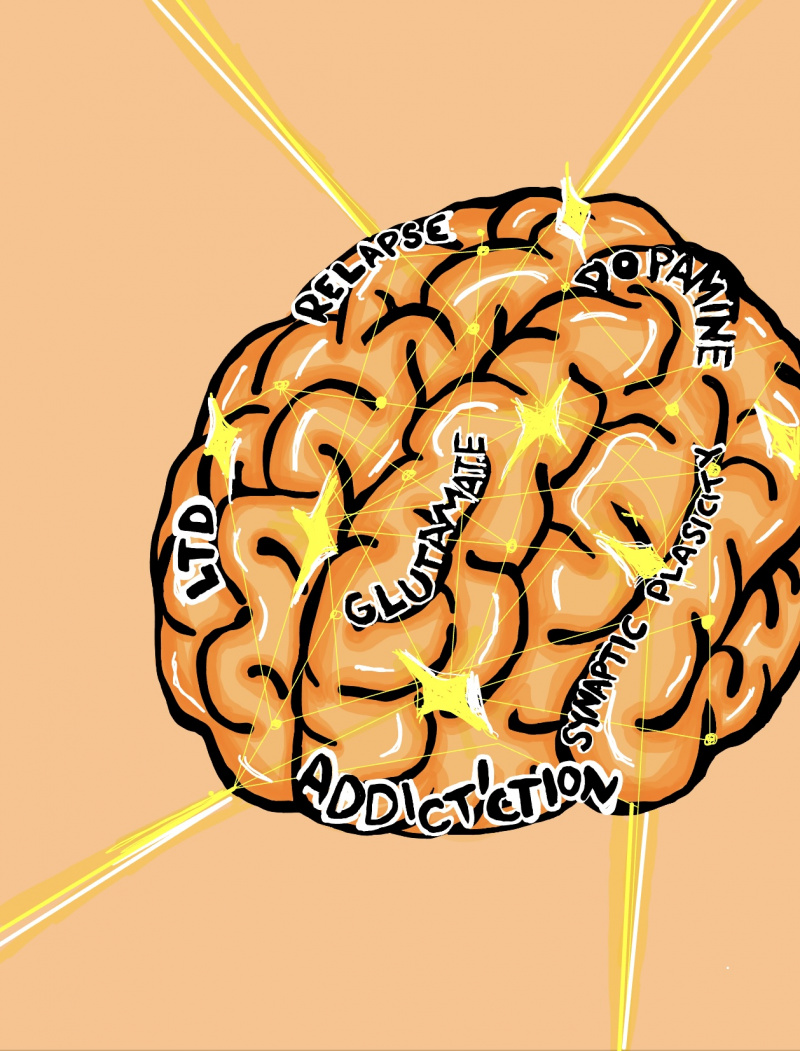
HIJACKED HAPPINESS: HOW ADDICTION REWIRES THE BRAINS REWARD SYSTEM
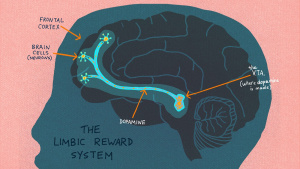
Addiction hijacks the brain’s reward circuits, reshaping the very wiring that governs pleasure and motivation. Key areas such as the ventral tegmental area (VTA), nucleus accumbens (NAc), prefrontal cortex (PFC), and amygdala—which are normally involved in reinforcing survival behaviors like eating or socializing—are overtaken by psychostimulants. These drugs flood the brain with dopamine, causing compulsive drug-seeking behaviors and diminishing the sensitivity to natural rewards. Glutamate, another neurotransmitter crucial for synaptic plasticity, is disrupted, leading to long-lasting changes that reinforce addiction and heighten vulnerability to relapse. By targeting neurotransmitter systems, innovative treatments can help restore balance in the reward circuitry, offering hope for those suffering from psychostimulant use disorder. [1]
WHAT IS PUD?
Psychostimulant Use Disorder (PUD) is a chronic, relapsing condition characterized by an uncontrollable drive to consume psychostimulants, even in the face of negative emotional and physical consequences. It causes profound neuroadaptations within the glutamatergic circuitry responsible for reinforcement and reward processing, resulting in physical and mental impairments. Psychostimulants include a range of substances such as nicotine, cocaine, methamphetamine, MDMA, dextroamphetamine, and methylphenidate—all of which trigger extensive changes in glutamate transmission and receptor function, playing a pivotal role in both the development and persistence of PUD.
THE ROLE OF THE REWARD CIRCUIT
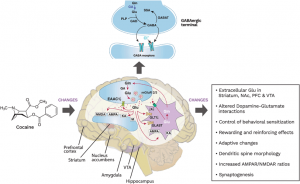
The brain’s reward system comprises interconnected regions—the VTA, NAc, PFC, hippocampus (HPC), and amygdala (AMY)—that work together to encourage survival behaviors like eating and socializing to give us feelings of pleasure and motivation. However, psychostimulants exploit these circuits, driving the transition from initial drug use to addiction. Dopaminergic neurons in the VTA regulate glutamate and GABA neurotransmission, ensuring balance in the brain’s signaling. When this balance is disrupted, addictive behaviors become deeply ingrained.[2] The interactions among various parts of this circuit and neuroadaptations in these areas mainly contribute in the rewarding impact of drugs and to the progression from initial drug use to PUD’s.
GLUTAMATE: THE MAIN NEUROTRANSMITTER
Glutamate is the brain’s most abundant excitatory neurotransmitter, making up about 70–90% of the brain’s synaptic communication. Glutamate helps neurons “talk” to each other quickly, which is essential for processes like learning, memory, and overall brain function.
Its role in addiction is profound, as glutamate projections from the prefrontal cortex (mPFC) and amygdala to the NAc directly influence GABAergic activity, which regulates dopamine transmission. Different glutamate receptors—including NMDA, AMPA, kainate, and metabotropic receptors (mGluRs)—are involved in addiction-related neuroadaptations, highlighting the complexity of glutamatergic involvement in psychostimulant use disorder. This ability to rapidly transmit signals also plays a key role in synaptic plasticity, the brain’s way of adapting and changing based on experiences.
Metabotropic glutamate receptors (mGluRs)—classified I, II, and III—play a key role in synaptic plasticity within brain reward circuitry. These G-protein coupled receptors are distributed throughout the peripheral nervous system and interact with psychostimulants such as cocaine, amphetamines, methamphetamine, and nicotine.
Beyond addiction, glutamate is vital for learning and memory. Changes in glutamate function not only influence recall and cognitive adaptability but also contribute to the development of addictive behaviors. Among the crucial receptors involved in synaptic plasticity and addiction are:
- NMDA and AMPA receptors: Facilitate fast excitatory signals.
- Kainate and metabotropic glutamate receptors (mGluRs): Engage in more intricate signaling processes.
SYNAPTIC PLASTICTY– ADDICTION
Addiction is more than physical dependency—it’s deeply intertwined with memory and learning processes, particularly in the hippocampus (HPC), the brain’s center for memory. A key concept that explains addiction’s impact on the brain is synaptic plasticity, which refers to the brain’s ability to strengthen or weaken connections between neurons over time. This dynamic process shapes how we learn, remember, and even how addiction memories persist—playing a critical role in relapse.
Types of Synaptic Plasticity:
- Structural Plasticity: This involves physical changes in the brain, like the formation or pruning of dendrites and synapses, which are the connections between neurons.
- Functional Plasticity: The brain’s ability to shift functions from damaged areas to healthier regions, allowing it to adapt to new situations.
In the case of addiction, the brain’s natural adaptability is exploited, creating powerful neural circuits that are tied to drug-seeking behaviors. Over time, addiction essentially “rewires” the brain’s reward and memory systems to prioritize substance use.[3]
HOW SYNAPTIC PLASTICITY DRIVES ADDICTION
- Repeated drug use reinforces synapses linked to drug-related cues, such as specific sights, smells, or environments. This process, largely driven by Long-Term Potentiation (LTP), occurs in key brain regions like the hippocampus (HPC), nucleus accumbens (NAc), and ventral tegmental area (VTA). Over time, these circuits become deeply ingrained, fueling compulsive drug-seeking behaviors.
- Drugs overwhelm the brain with dopamine, dulling its responsiveness to everyday pleasures like eating or socializing. Long-Term Depression (LTD) affects neural circuits linked to natural rewards, effectively rewiring the brain to prioritize substance use over other activities.
WHAT IS LTP? LONG TERM POTENTIATION
LTP is the process by which synaptic connections are strengthened after repeated stimulation. It’s crucial for learning and forming long-lasting memories. LTP helps solidify the connection between drug-related cues (like people, places, or paraphernalia) and the pleasurable high that comes with using substances. This means that even after a period of abstinence, these “reinforced” memories can quickly resurface when exposed to these triggers, increasing the risk of relapse.
WHAT IS LTD? LONG TERM DEPRESSION
LTP is the weakening of synaptic connections after low-frequency stimulation. It allows the brain to “forget” less relevant information over time. Addiction shows that LTD can suppress memories tied to natural rewards, like the joy of spending time with loved ones. Over time, this creates an imbalance where drug-related rewards take precedence, further cementing the addictive behavior and making it harder to return to normal, healthy reward patterns. [4]
NEUROPLASTICITY FOR RECOVERY
The psychostimulant-induced behavioral and neurological plasticity could potentially explore circuit and molecular targets with the potential to contribute to the treatment of PUD. To date, there are no FDA-confirmed medicines for the treatment of psychostimulant abuse; therefore, clarification of the cellular and molecular alterations participating in PUD is crucial for developing beneficial medications.
Short- and long-term adaptive changes in dopamine transmission and brain circuitry have been extensively studied as mediators of PUD and its associated neuropsychiatric impairments. These studies underline the importance of neurotransmitter synthesis and release in various parts of the reward circuit, which are significantly altered by prolonged drug use. Such disruptions are primary contributors to the cognitive and behavioral impairments seen in individuals with PUD.
Innovative treatments targeting glutamate receptors or excitatory amino acid transporters (EAATs) offer promising pathways to normalize synaptic transmission and reduce cravings. By restoring balance within the reward circuitry and addressing neuroplastic changes, these therapeutic approaches could play a critical role in reshaping the future of addiction recovery.

REFERENCES
Cooper, S., Robison, A. J., & Mazei-Robison, M. S. (2017). Reward Circuitry in Addiction. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics, 14(3), 687–697. https://doi.org/10.1007/s13311-017-0525-z
Kalivas, P. W., Lalumiere, R. T., Knackstedt, L., & Shen, H. (2009). Glutamate transmission in addiction. Neuropharmacology, 56 Suppl 1(Suppl 1), 169–173. https://doi.org/10.1016/j.neuropharm.2008.07.011
Mozafari, R. et al, (2023) A review on the role of metabotropic glutamate receptors in neuroplasticity following psychostimulant use disorder, Progress in Neuro-Psychopharmacology and Biological Psychiatry,Volume 124. https://doi.org/10.1016/j.pnpbp.2023.110735
Puderbaugh, M. (2023, May 1). Neuroplasticity. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK557811/
FOOTNOTES
[1] Kalivas, P. W., Lalumiere, R. T., Knackstedt, L., & Shen, H. (2009). Glutamate transmission in addiction. Neuropharmacology, 56 Suppl 1(Suppl 1), 169–173. https://doi.org/10.1016/j.neuropharm.2008.07.011
[2] Cooper, S., Robison, A. J., & Mazei-Robison, M. S. (2017). Reward Circuitry in Addiction. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics, 14(3), 687–697. https://doi.org/10.1007/s13311-017-0525-z
[3] Puderbaugh, M. (2023, May 1). Neuroplasticity. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK557811/
[4] Mozafari, R. et al, (2023) A review on the role of metabotropic glutamate receptors in neuroplasticity following psychostimulant use disorder,Progress in Neuro-Psychopharmacology and Biological Psychiatry,Volume 124.https://doi.org/10.1016/j.pnpbp.2023.110735