Trauma’s Code: Stress Signals and The Epigenetic Blueprint
Stress is a universal human experience, yet its impact on the brain often goes unnoticed. It leaves a lasting molecular and cellular footprint, shaping emotions, behaviors, and memories. By unraveling the science behind stress responses, we can better understand how stress rewires the brain and explore the critical roles of neurotransmitters and epigenetic changes in trauma recovery.
Understanding PTSD, Anxiety, and Stress
Anxiety and stress disorders are widely prevalent among individuals with PTSD, producing emotional and physiological responses that affect overall well-being. The interactions between stress and emotional or cognitive processing can reveal how such mechanisms contribute to the development and persistence of PTSD and stress.
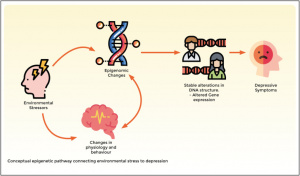
The process of converting DNA instructions into RNA is fundamental for driving cellular responses; in this case of stress, glucocorticoid hormones are involved, which play a pivotal role in regulating the brain’s stress response and the functionality of the hippocampus, which is a region essential for memory and emotional regulation.
GABAergic neurons are crucial, which regulate brain excitability, and the limbic brain structures, including the amygdala and hippocampus, as the emotional core of the brain.
Finally, the role of vital signaling pathways, such as ERK-MAPK, and transcription factors like CREB, emerges as essential contributors to the molecular changes induced by stress. As shown in Fig.2.
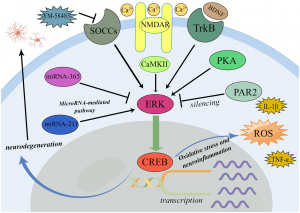
What is an Epigenetic Pathway?
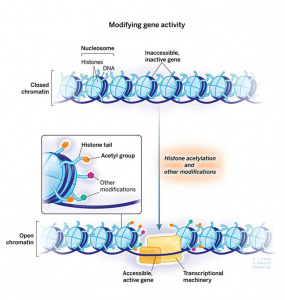
Epigenetic pathways are powerful mechanisms that regulate how stress impacts the brain, without altering the underlying DNA sequence. These processes involve modifications to histones, which are proteins that DNA wraps around. As well as chemical additions like methylation or acetylation, which influence whether specific genes are “switched on” or “off.”
In the context of stress and PTSD, epigenetic changes can increase or suppress gene expression, particularly in areas like the hippocampus and amygdala. For example, stress-induced histone modifications, shown in Fig. 3 such as serine10 phosphorylation and lysine14 acetylation, play a role in activating immediate early genes (IEGs) critical for neural adaptation and behavioral responses. [4]
These epigenetic marks have effects across signaling pathways like ERK-MAPK, reshaping how the brain responds to fear and anxiety over time. Such changes can be enduring, encoding trauma and contributing to long-term vulnerability to PTSD, The dynamics between stress and the epigenetic marks emphasize the brain’s remarkable adaptability and synaptic plasticity. [5]
Acute Stress vs Chronic Stress
- Acute stress can sometimes be beneficial, as it primes the brain for quick responses. However, chronic stress is a significant risk factor for anxiety disorders.
- Chronic stress or PTSD induce changes in the amygdala, PFC, and hippocampus which contribute to heightened anxiety, impaired emotional regulation, and difficulty distinguishing between real and perceived threats.

Acute Stress
Acute stress is short-term and typically arises in response to immediate threats or challenges, such as a sudden deadline or a near-miss car accident.
The brain’s amygdala detects the threat and signals the hypothalamus, which activates the sympathetic nervous system. This triggers the “fight-or-flight” response, releasing stress hormones like adrenaline and cortisol.
Effects on the Brain:
- Enhancement of glutamate release in the PFC, improving focus and decision-making temporarily.
- Activates the hippocampus, aiding memory formation related to the stressful event.
- Once the stressor is resolved, the parasympathetic nervous system restores balance, reducing cortisol levels.[1]
Chronic Stress
Chronic stress occurs when stressors persist over time, such as ongoing financial difficulties or a toxic work environment.
This causes prolonged activation of the hypothalamic-pituitary-adrenal (HPA) axis , which then leads to sustained cortisol release.
Affects on the Brain
- Hippocampus: Chronic cortisol exposure can cause atrophy of hippocampal neurons, impairing memory and learning. Chronic cortisol causes dendritic shrinkage and spine loss, impairing episodic memory and spatial navigation.
- Prefrontal Cortex: Reduced activity in the PFC weakens decision-making and emotional regulation. Dendrites in the medial PFC retract, impairing memory and emotional resilience, while the orbitofrontal cortex (OFC) shows dendritic expansion associated with hypervigilance.
- Amygdala: Becomes hyperactive, heightening fear and anxiety responses. Acute stress increases dendritic growth temporarily, but chronic stress leads to more enduring expansions in basolateral amygdala (BLA) dendrites, heightening anxiety and fear responses.
- Neuroplasticity: Chronic stress disrupts synaptic plasticity, leading to long-term changes in brain circuits associated with anxiety and depression. [2]
| Acute Stress | Chronic Stress |
| Short-term HPA axis activation. | Prolonged HPA axis activation and dysregulation. |
| Boosts PFC activity for focused thinking. | Impairs PFC function, weakening decision-making and emotional regulation. |
| Enhances hippocampal memory encoding. | Causes hippocampal atrophy and reduced neurogenesis. |
| Balanced glutamate levels support plasticity. | Excess glutamate triggers excitotoxicity. |
| Parasympathetic system restores balance. | Persistent cortisol alters brain circuits. |
Stress and Structural Changes in The Brain
Anxiety and stress disorders are common among individuals with PTSD, producing emotional and physiological responses that can affect overall well-being. Critical aspects include gene transcription, the process of converting DNA instructions into RNA to drive cellular responses, and the role of glucocorticoid hormones, which influence the brain’s ability to respond to stress. The adrenal cortex produces corticosterone, which is a vital glucocorticoid hormone involved in the body’s stress response. As shown in Fig. 5
Corticosterone plays a central role in regulating metabolism, immune response, and the HPA axis. When the body encounters stress, the adrenal cortex releases corticosterone into the bloodstream to help maintain homeostasis. This hormone influences the energy balance by increasing glucose availability, ensuring that the body has enough resources to respond to the stressor.
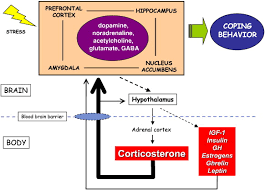
Additionally, the hippocampus, is highlighted alongside GABAergic neurons that manage brain excitability through the inhibitory neurotransmitter GABA. Furthermore, limbic brain structures such as the amygdala and hippocampus, as well as critical signaling pathways like ERK-MAPK and transcription factors such as CREB, play a significant role in the stress-induced molecular changes.
Neurotransmitters Involved in Stress
- Glutamate: As the brain’s primary excitatory neurotransmitter, glutamate enables rapid communication between neurons. Acute stress uses glutamate effectively to enhance cognitive performance, but chronic stress floods the brain, causing excitotoxicity and disrupting synaptic plasticity. Glutamate is critical for associative learning and emotional processing under stress. Dysregulated glutamate signaling contributes to distortions in how PTSD patients process information. [3]
- GABA (Gamma-Aminobutyric Acid): The brain’s primary inhibitory neurotransmitter, GABA helps counterbalance glutamate’s excitatory effects, calming hyperactive neurons. Chronic stress reduces GABA function, heightening anxiety and emotional reactivity.
- Dopamine: Acute stress briefly boosts dopamine, aiding motivation and focus. Chronic stress depletes dopamine, contributing to loss of pleasure and depression.
- Serotonin: Regulates mood and emotional processing. Stress can diminish serotonin levels, increasing vulnerability to anxiety and depression.
- Noradrenaline (Norepinephrine): Heightens alertness and attention during acute stress but can lead to heightened vigilance and overreaction under chronic stress.
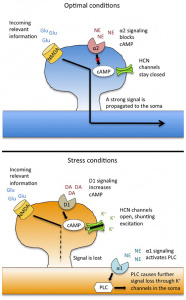
These neurotransmitters form the biochemical foundation for how the brain processes and adapts to stress, playing a critical role in shaping behaviors and emotional outcomes in PTSD. As seen in Fig. 6 Optimal conditions versus stressful conditions in a neurotransmitter.
Why This Matters
Stress and PTSD leave lasting imprints on the brain, from changes in neurotransmitter function to epigenetic marks that shape how we process fear, memory, and resilience. We can understand the brain’s plasticity allows it to adapt to stress, but also how chronic stress can disrupt this balance, leading to vulnerabilities like anxiety, depression, and PTSD.
Understanding the molecular and cellular effects of stress gives us more than knowledge, it gives the potential future of treatment. By targeting pathways like ERK-MAPK or leveraging therapeutic potential in neurotransmitters such as GABA and glutamate, science paves the way for innovative treatments that go beyond symptom management to address the root causes of trauma.
REFRENCES
Gudsnuk, K., & Champagne, F. A. (2012). Epigenetic influence of stress and the social environment. ILAR journal, 53(3-4), 279–288. https://doi.org/10.1093/ilar.53.3-4.279
Howie, H., Rijal, C. M., & Ressler, K. J. (2019). A review of epigenetic contributions to post-traumatic stress disorder . Dialogues in clinical neuroscience, 21(4), 417–428. https://doi.org/10.31887/DCNS.2019.21.4/kressler
JM;, R. (n.d.). Making memories of stressful events: A journey along epigenetic, gene transcription, and signaling pathways. Frontiers in psychiatry. https://pubmed.ncbi.nlm.nih.gov/24478733/
Martin, E. I., Ressler, K. J., Binder, E., & Nemeroff, C. B. (2009). The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. The Psychiatric clinics of North America, 32(3), 549–575. https://doi.org/10.1016/j.psc.2009.05.004
FOOTNOTES
[1] JM;, R. (n.d.). Making memories of stressful events: A journey along epigenetic, gene transcription, and signaling pathways. Frontiers in psychiatry. https://pubmed.ncbi.nlm.nih.gov/24478733/
[2] JM;, R. (n.d.). Making memories of stressful events: A journey along epigenetic, gene transcription, and signaling pathways. Frontiers in psychiatry. https://pubmed.ncbi.nlm.nih.gov/24478733/
[3] Martin, E. I., Ressler, K. J., Binder, E., & Nemeroff, C. B. (2009). The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. The Psychiatric clinics of North America, 32(3), 549–575. https://doi.org/10.1016/j.psc.2009.05.004
[4] Howie, H., Rijal, C. M., & Ressler, K. J. (2019). A review of epigenetic contributions to post-traumatic stress disorder . Dialogues in clinical neuroscience, 21(4), 417–428. https://doi.org/10.31887/DCNS.2019.21.4/kressler
[5] Gudsnuk, K., & Champagne, F. A. (2012). Epigenetic influence of stress and the social environment. ILAR journal, 53(3-4), 279–288. https://doi.org/10.1093/ilar.53.3-4.279
