The article “Insulin Signaling Pathway and Related Molecules: Role in Neurodegeneration and Alzheimer’s Disease” explores how insulin dysfunction contributes to the development of neurodegenerative disorders, particularly Alzheimer’s Disease (AD). It highlights key pathways, including insulin resistance, neuroinflammation, and oxidative stress, and their roles in neuronal damage.
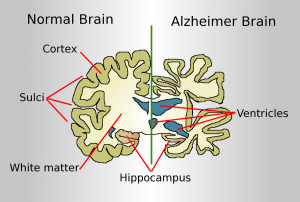
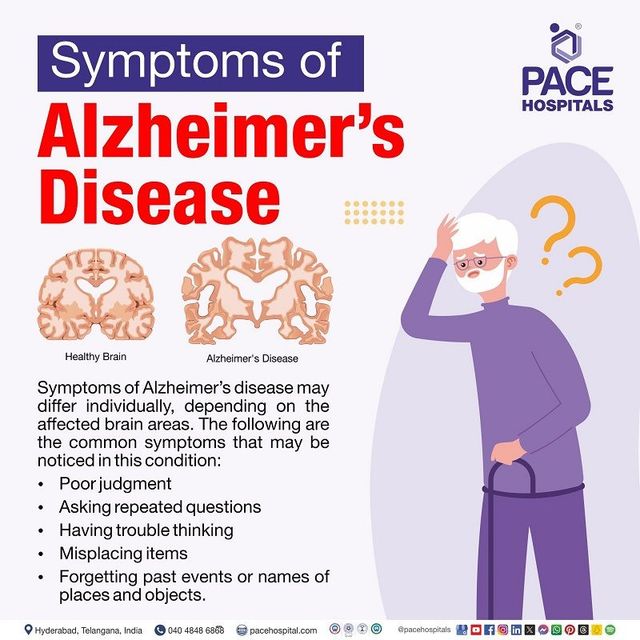
Neurodegenerative illnesses, particularly Alzheimer’s disease (AD), are an increasing public health concern, affecting millions of people worldwide. Understanding and addressing the molecular pathways that cause neuronal damage is critical for reducing these disorders. One such crucial mechanism is the Nrf2 (Nuclear Factor Erythroid 2-Related Factor 2) signaling system, which is the primary regulator of the body’s antioxidant response. According to emerging research, Nrf2 has an important role in countering oxidative stress and insulin resistance, both of which contribute to neurodegeneration and cognitive decline. Scientists hope that by unleashing Nrf2’s therapeutic potential, they will be able to develop therapies that will reduce or prevent diseases like Alzheimer’s.
Nrf2 is a transcription factor that controls the production of numerous antioxidant and detoxification enzymes, thereby protecting cells from oxidative damage. Under normal physiological settings, Keap1 (Kelch-like ECH-associated protein 1) inhibits Nrf2, allowing it to degrade. However, when exposed to oxidative stress, Nrf2 is produced, translocates to the nucleus, and activates protective genes . According to research, dysregulated Nrf2 signaling leads to Alzheimer’s disease progression by aggravating oxidative stress and increasing insulin resistance. Studies have indicated that a drop in Nrf2 activity is connected with increased oxidative damage in neurons, resulting in protein misfolding, neuroinflammation, and eventually neuronal death . Furthermore, Nrf2 has been shown to interact with insulin signaling pathways. Inhibiting Nrf2 decreases the phosphorylation of Akt, which is a crucial regulator of glucose metabolism and neuronal survival. This disruption adds to insulin resistance, which is not just a hallmark of diabetes but also connected to cognitive decline and Alzheimer’s disease progression.
How Do We Activate Nrf2 for Brain Health?
Given its protective effect, therapeutic stimulation of Nrf2 is a promising therapy for neurodegenerative disorders. Several approaches include:
- Pharmacological activation: Certain substances, including as sulforaphane (found in broccoli), curcumin (from turmeric), and synthetic Nrf2 activators, can boost Nrf2 signalling and enhance antioxidant defenses.
- Regular exercise, intermittent fasting, and a polyphenol-rich diet can improve Nrf2 activation and cellular resilience against oxidative stress
- Inhibiting GSK-3β may boost Nrf2 activation, making it a possible therapeutic target for Alzheimer’s disease.
Insulin signaling pathway
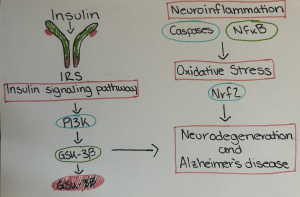
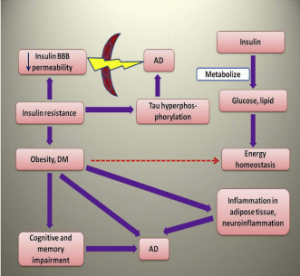
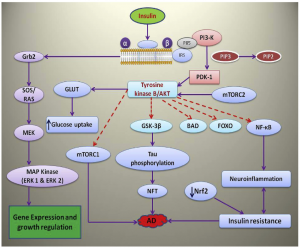
Insulin signaling pathway and the role of its molecules in neurodegeneration and AD has been described in the following paragraphs Fig. 1 . 
Fi.1. Role of insulin and its binding to receptor, role of various molecules in the insulin signaling pathway and subsequent role in neurodegeneration and Alzheimer’s disease.
Nrf2 and Insulin Signaling
Nuclear factor erythroid-2-related factor 2 (Nrf2) is a basic leucine protein that regulates antioxidant protein expression. Cells express it ubiquitously and constitutively. Under normal physiological settings, it is constantly destroyed by the ubiquitin proteasome system, and low Nrf2 levels result in basal expression of its target genes. The E3 ligase adaptor Kelch-like ECH-associated protein 1 (KEAP 1) regulates Nrf2 stability
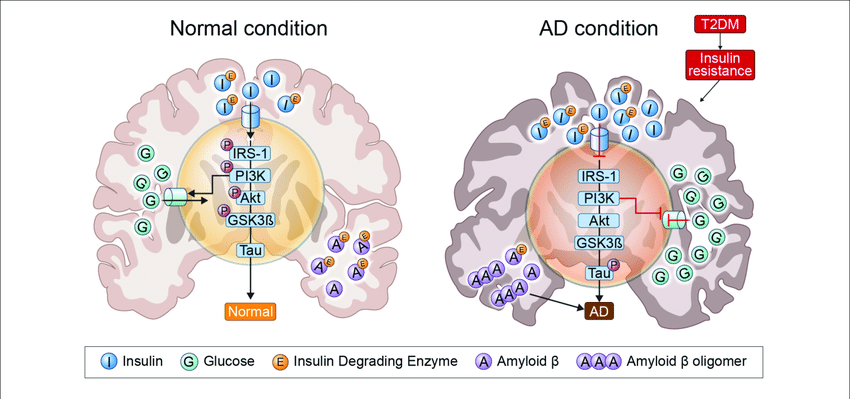
Nrf2 is a novel and very promising target in many neurodegenerative diseases including AD. Neurodegeneration involving oxidative stress and insulin resistance can be alleviated by targeting the Nrf2 pathway. Inhibitors of GSK3 can activate Nrf2 signaling as they are likely to blunt βTrcP-mediated degradation and impede nuclear exclusion of Nrf2. The same applies to compounds activating MAPK (ERK, JNK, and p38) signaling( Zhang et al., 2014) (Fig. 2).

Fig. 2. Involvement of Nrf2 in oxidative stress and insulin resistance through keap1, GLUT4, PI3K/Akt.
With an aging global population, the burden of neurological illnesses is likely to increase. Targeting the Nrf2 pathway could lead to significant advances in neuroprotection and disease prevention. By deepening our understanding of this system, we can pave the road for novel treatments that may one day slow or even reverse cognitive decline. Incorporating Nrf2-boosting techniques, such as dietary changes and exercise, could be a proactive approach to brain health. Furthermore, ongoing investment in research and clinical trials is critical for converting these results into viable medicines.
In a nutshell, malfunction and anomalies in the insulin signaling system and related components may contribute to the neurodegenerative process that characterizes Alzheimer’s disease. Several studies found that manipulation of components downstream in the insulin signaling cascade enhanced insulin sensitivity. It can also be concluded that there is a substantial relationship between neuroinflammation and insulin resistance. Diabetes mellitus and Alzheimer’s disease share characteristics such as insulin resistance and inflammation. Furthermore, insulin resistance has been linked to other neurodegenerative disorders such as Parkinson’s and Huntington’s disease.
As a result, from a therapeutic standpoint, improving the insulin signaling system by modulating its components can slow neurodegeneration and alleviate illness symptoms.
References
- Akhtar, A., & Sah, S.P. (2020). “Insulin signaling pathway and related molecules: Role in neurodegeneration and Alzheimer’s disease.” Neurochemistry International. DOI: 10.1016/j.neuint.2020.104707.
- Morrison, C.D., et al. (2010). “High-fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling.” Journal of Neurochemistry. DOI: 10.1111/j.1471-4159.2010.06609.x.
- Suzuki, T., & Yamamoto, M. (2015). “Molecular basis of the Keap1-Nrf2 system.” Free Radical Biology and Medicine. DOI: 10.1016/j.freeradbiomed.2014.12.017
- Park, J.G., et al. (2016). “Indirect activation of Nrf2 by AMPK and PI3K/Akt signaling pathways.” Antioxidants & Redox Signaling. DOI: 10.1089/ars.2016.6778










 [1]
[1]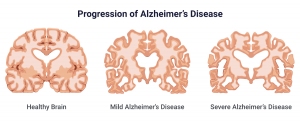 [5]
[5]