A. The Paper
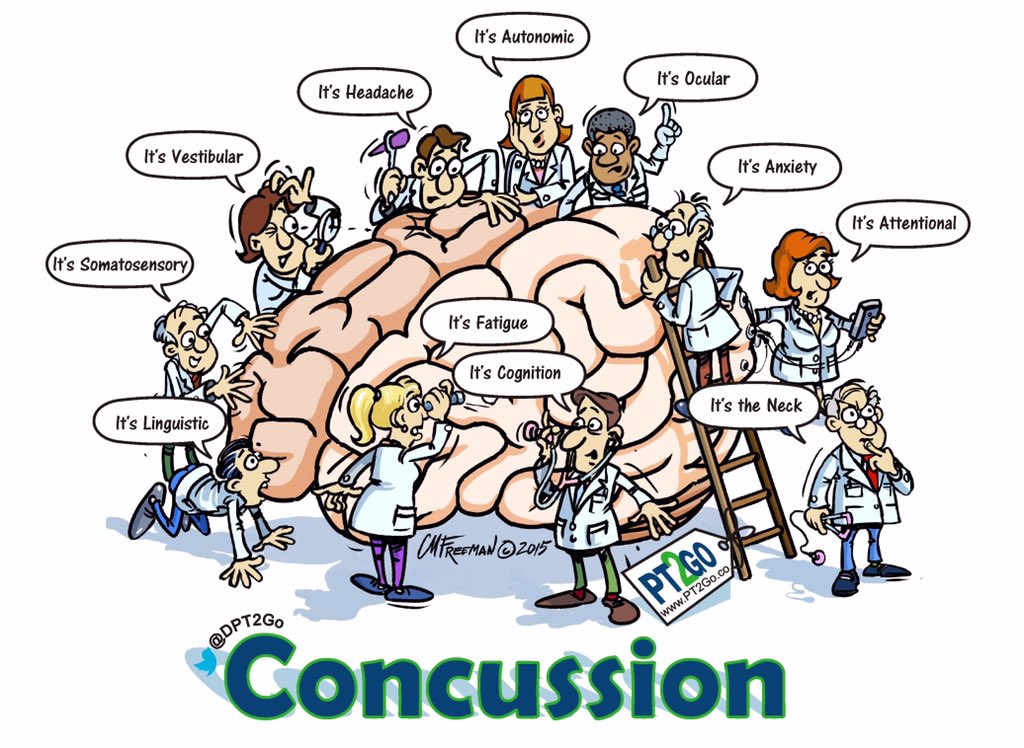
The New Neurometabolic Cascade of Concussion looks into the evolving understanding of the pathophysiological mechanisms underlying concussions, shedding light on a paradigm shift in how these injuries are conceptualized and managed.
Traditionally, concussions have been viewed as purely functional disturbances, characterized by transient neurological dysfunction without structural damage. However, Giza and Hovda propose a new perspective, suggesting that concussions involve complex neurometabolic cascades that can lead to long-lasting consequences.
B. Steps of concussion
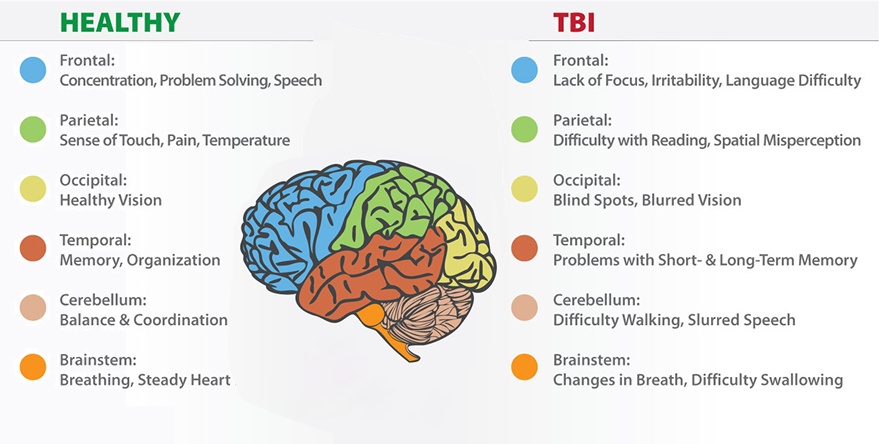
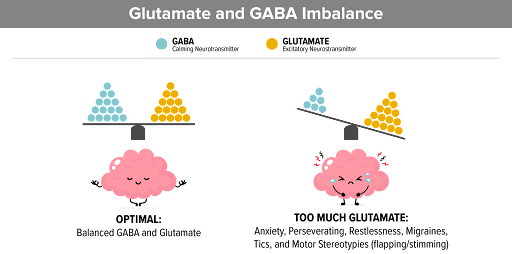
The paper outlines a multi-step cascade of events triggered by concussive impacts, starting with the immediate disruption of ion gradients and neurotransmitter release. This initial phase is followed by a metabolic crisis, characterized by increased energy demands and compromised cerebral blood flow regulation. As a result, neurons experience energy depletion and mitochondrial dysfunction, leading to oxidative stress and cellular damage.
Moreover, the role of neuroinflammation is highlighted in the secondary injury phase of concussions, where activated microglia release inflammatory mediators and exacerbate neuronal damage. This inflammatory response can persist for days to weeks following the initial injury, contributing to ongoing neurological dysfunction and potential long-term consequences.
C. Imaging

There was a large interest in imaging tools that can be used to diagnose concussions. My part was about fMRI. A Functional Magnetic Resonance Image (fMRI) is an imaging technique used to diagnose concussion and recognize changes in the brain while you are asked to engage in cognitive tasks.
D. Recovery and Being in School
The paper emphasizes the importance of considering individual variability in concussion outcomes, noting that factors such as age, sex, genetic predisposition, and prior injury history can influence the severity and duration of symptoms. It also stresses the need for personalized approaches to concussion management, including targeted interventions aimed at mitigating specific aspects of the neurometabolic cascade.
Furthermore, the paper discusses the implications of their proposed model for concussion diagnosis, treatment, and prevention. They advocate for a comprehensive approach that integrates clinical assessment, neuroimaging, biomarker analysis, and neuropsychological testing to better understand and manage concussive injuries.
I found this website from the University of Michigan particularly helpful for the treatment and recovery information, especially if you are still in school: Concussion Treatment and Recovery
Reference: