There is a great deal of stigmatism about weight in the United States. While there is some media about self-acceptance and building confidence we constantly encounter a barrage of messages (whether intentional or not) through adds, television, magazines, the internet, celebrities that show unrealistic body images. Articles in magazines emphasize quick fix weight loss on the front cover along with celebrities in scant clothing or stories about how someone famous gained 20 pounds (The horror! The horror!). I feel like I see advertisements such as

Every time I’m on the internet. And we do have unrealistic expectations.
| Average U.S. Woman | Barbie Doll | Store Mannequin | |
| Height | 5’4’’ | 6’0’’ | 6’0’’ |
| Weight | 145 lbs. | 101 lbs. | N/A |
| Dress size | 11-14 | 4 | 6 |
| Bust | 36-37’’ | 39’’ | 34’’ |
| Waist | 29-31’’ | 19’’ | 23’’ |
| Hips | 40-42’’ | 33’’ | 334’’ |
Sonenklar, Carol. 2011. Anorexia and Bulimia (USA Today Health Reports: Diseases and Disorders. ) Minneapolis, MN: Twenty-First Century Books.
It’s no wonder that rates of eating disorders continue to increase. A little over a year ago one of the girls I babysat for in high school was diagnosed with anorexia. She is 12 years old. This is when eating disorders got real for me.
First, a few factoids about eating disorders
- An eating disorder is characterized by abnormal eating patterns that attempt to satisfy a psychological rather than physical need. The three most common disorders are anorexia nervosa, bulimia nervosa, and binge eating disorder. Anorexia nervosa is characterized by self-starvation, weight loss, an irrational fear of gaining weight, and a distorted body image. Bulima nervosa is characterized by a cycle of compulsive binging followed by purging through various means, such as vomiting, laxative/diuretic abuse, and extreme exercising. Binge eating disorder is the most common disorder and is characterized by frequent periods of compulsive overeating without accompanying purging behaviors.[i]
- Nearly 10 million females and 1 million males have a form of anorexia or bulimia in the United States. Millions more are struggling with compulsive eating disorder. Additionally, over 70 million people worldwide struggle with an eating disorder.[ii]
- Forty percent of new cases of anorexia are in girls between the ages of 15 and 19.[iii]
Marijuana in the Brain
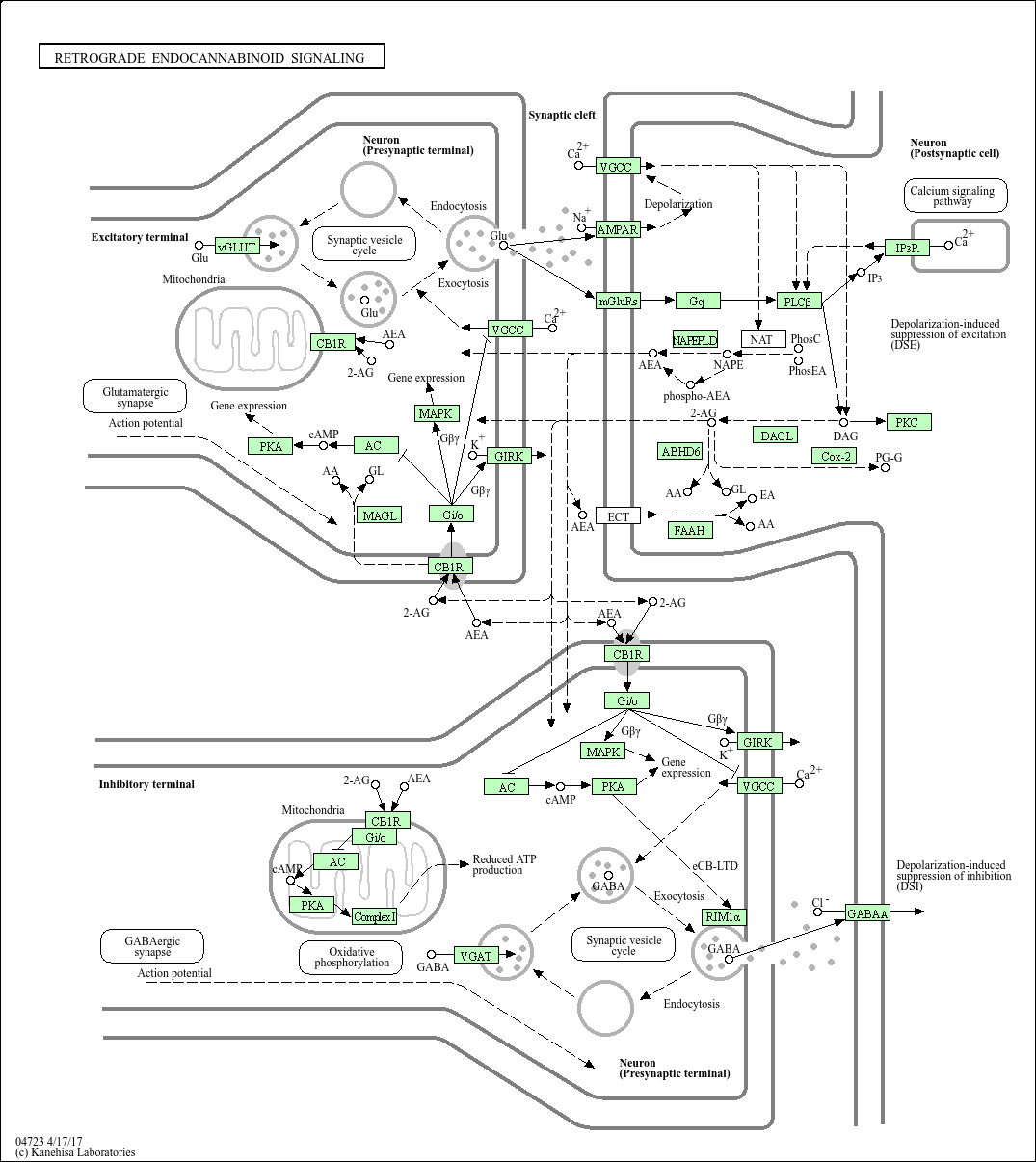
Marijuana has 2 active chemicals: tetrahydrocannabinol (THC) and cannabidiol, both of which are linked in the endocannabinoid neurotransmitter system. The endocannabinoid system works backwards from many other neurotransmitters because it releases signals from the post-synaptic neuron to the CB1 and CB2 receptors on the pre-synaptic neuron as shown in the diagram below.
THC has been related through studies to the psychoactive, munchies, addictive qualities of marijuana and in high doses can cause or increase anxiety. There have also been studies that show that endocannabinoids interact with the dopamine system, playing a role in drug taking and seeking behaviors. [iv] Cannabidiol has been related to the therapeutic effects which is why marijuana is used in cancer treatments,pain relief, and has also been used as a treatment for schizophrenia.
Marijuana as a treatment for eating disorders
Marijuana has also been used as a way to help AIDS patients maintain a healthy weight as an effect of their disease they lose appetite and stop eating. This is part of the premise of using marijuana as a way to help gain or lose weight because of it’s link to the reward system and studies have shown that endocannabinoids CB1 receptors in the hypothalamus play a large role in food intake. Obviously, this is a very simplified synopsis of how the endocannabinoid system could be linked in a way that would help with the treatment of eating disorders. There is a lot that is still unknown about the endocannabinoid system and all of the parts of the brain of which it is an integral part. One of the objections that one could make about using marijuana as a medical treatment is that it is not specific enough to the effected brain area. Even if scientists were able to create synthetic products to emulate the positive effects of the drug without the addictive or psychoactive effects, there is currently no way to target specifically the hypothalamus, if that is the only part of the brain that is needed to be targeted with eating disorders. Is there enough known about the diseases or the endocannabinoid system to use it as a treatment? Another part to take into consideration is that a large part of the disease is about body image and perception, and while the treatment may help patients gain weight or change some stimuli of eating to positive, is that going to help with body image?
The legalization of marijuana is a complicated subject and there is a lot that we do not know about how the drug effects out brain. It has been shown to have therapeutic effects, whether or not those effects can help with eating disorder treatment is also very complicated and could use more research.
If you or someone you know have an eating disorder or you would like more information about eating disorders visit http://www.nationaleatingdisorders.org/