As the semester draws to a close, I find myself reflecting not only on the intricate mechanisms of neurotransmission, psychotropic medications, and molecular pathways we’ve explored in Neurochemistry, but also on the broader educational journey that brought me here. This final blog post is more than a conclusion to a course – it’s a culminating reflection of how my time at Concordia, shaped by the CORE curriculum and liberal arts foundation, has helped me become more critically aware, deeply curious, and responsibly engaged in the world.
A Love for Learning – Ignited and Sustained
One of the greatest gifts of this course, and my education at Concordia more broadly, has been the way it has deepened my love for learning. Neurochemistry is not an easy subject, especially when you have not partaken in a prerequisite course for the class. This subject demands resilience, a willingness to grapple with complexity, and a constant push to connect micro-level mechanisms with macro-level behaviors. But it was in that challenge that I found genuine excitement. I remember the first time we traced the role of serotonin from biosynthesis to its impact on mood regulation. Suddenly, abstract knowledge had tangible emotional resonance.
This kind of joy in the learning process is something that has been nurtured across disciplines here. Whether through analyzing behavior in a psychotherapy lab, exploring cultural perspectives in my liberal arts seminars, or working directly with clients as a Direct Support Professional at CCRI, I’ve been encouraged to lean into curiosity. Neurochemistry, specifically, allowed men to bring curiosity to the intersection of biology and behavior, a space that perfectly aligns with my passion for understanding the human experience.
:max_bytes(150000):strip_icc()/government-job-profile-direct-support-professional-1669627-final-24f90e7cdcb841c3b2954fa4997613dd.png)
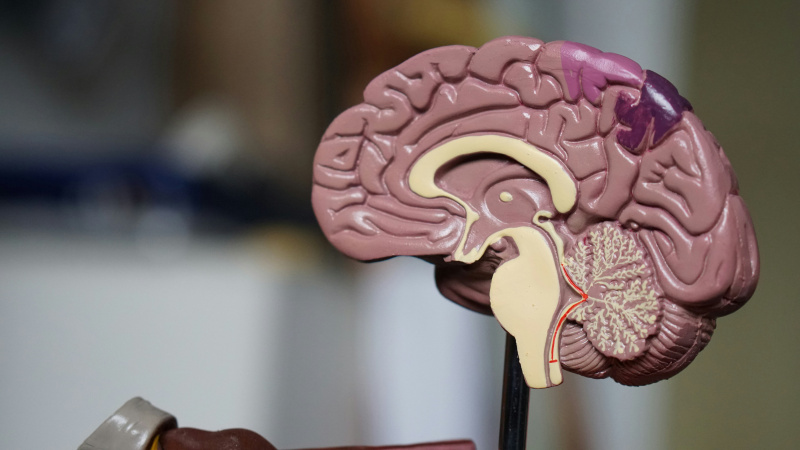
Figure 1 [1]
Foundational Skills & Transferable Capacities
While the content of Neurochemistry is specialized, the skills I’ve developed through it are transferable and wide-reaching. This course required me to engage in critical reading of complex scientific literature, to synthesize large volumes of information, and to communicate my findings clearly to writing and presentations. Perhaps more importantly, it sharpened my analytical reasoning. Neurochemistry forces you to think like a detective – interpreting data, evaluating hypotheses, and challenging assumptions.
One competency I’d highlight on my resume is, scientific communication. Though it still needs some work, this semester, I improved significantly in translating complicated biochemical concepts into language that is accessible to different audiences. Whether explaining neurotransmitter pathways to classmates from differing educational backgrounds or writing weekly blog posts, I learned how to distill complexity without oversimplifying.
This is a skill I hope to carry into a future career working with children affected by chronic illness. As a prospective family life specialist or therapist, I know that being able to explain medical and psychological information clearly and empathetically is essential – not just for patients, but for families, schools, and communities.

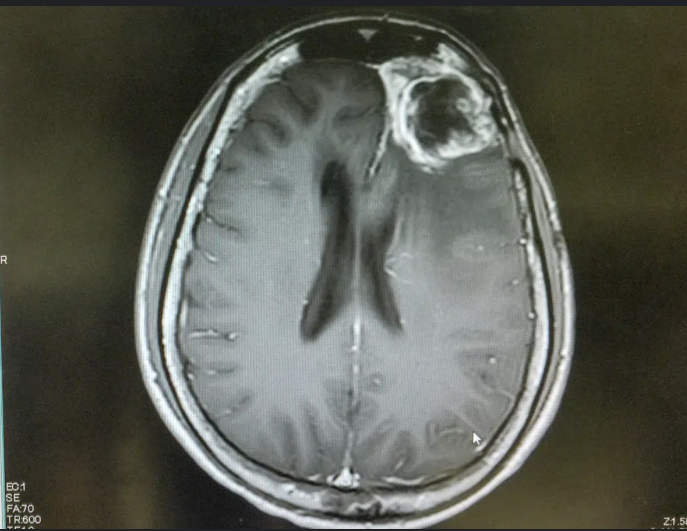
Figure 2 [2]
Learning Across Disciplines: Psychology, Neuroscience, and Beyond
One of the most enriching aspects of Neurochemistry was the way it naturally required interdisciplinary thinking. With my major in psychology and a minor in neuroscience, I was constantly toggling between lenses – viewing behavior as both a product of brain chemistry and of environmental context. Our discussion on depression, for instance, asked us to consider not just the serotonin hypothesis, but also cognitive-behavioral models, social determinants of health, and ethical implications of pharmaceutical intervention.
A moment that particularly stands out was when we analyzed the neurochemical basis of Autism, ADHD, and Anxiety – topics that are close to me personally. It was powerful to draw form multiple disciplines: understanding the pharmacology of stimulates and other drugs, reflecting on the psychological theories of executive function, and thinking about broader societal narratives around socialization, attention, and stigma. This was liberal learning in action: integrating scientific, ethical, and humanistic perspectives to better understand a complex issue.

Figure 3 [3]
Developing the Whole Self
Concordia’s goal to cultivate an examined cultural, ethical, physical, and spiritual self-understand has been a guiding force in my academic and personal growth. This course challenged me to consider the ethics of scientific advancement. When we discussed neuroenhancement or the use of SSRIs in young children, we weren’t just learning the mechanisms – we were confronting real-world dilemmas that required reflection on values and identity.
It also prompted internal dialogue. As someone who has experienced chronic illness and wrestled with mental health and ADHD, learning about neurochemistry was more than academic – it was personal. I found myself asking, “What does it mean to be ‘neurotypical’? How do we define ‘treatment’? Who gets access to it, and who is left behind?” These question pushed me to think more deeply about my future role in healthcare – not just as a provider of knowledge, but as an advocate for equity, empathy, and dignity.
Learning as Liberal Arts: A meaningful Integration
Learning at a liberal arts institution like Concordia has meant that I never had to choose between being a scientist and being a humanist. I could study neurotransmission and still attend a lecture on Indigenous perspectives in healthcare. I could research the brain and also volunteer in the community, engaging with real people facing real challenges. That blend – of heart and head, theory and practice – is what makes this place special.
Neurochemistry, as one of my final upper-level science courses, felt like the perfect capstone to this journey. It demanded scientific rigor but also invited philosophical reflection. It taught me about the Brian but also asked me to think about the mind, about society, and about my role in making the world a little better.
An Interdisciplinary Problem-Solving Example
One of the most compelling interdisciplinary experiences this semester was during our project on substance use and dopamine. To fully understand the topic, we pulled in pharmacology to understand receptor activity, psychology to explore addiction behaviors, and even sociology to examine how social environments shape drug use patterns.
The group I spoke with tackled the question: Why do some people become addicted while others don’t – even when exposed to the same substance? WE looked at genetic polymorphisms in dopamine receptors, considered comorbid mental health diagnoses, and explored economic and racial disparities in addiction treatment access. It was only by combining insights form multiple disciplines that we were able to offer a holistic perspective – and propose better intervention strategies. This kind of integrated problem – solving is something I hope to carry forward in my career.
Figure 4 [4]
Looking Forward: Skills, Dreams, and Responsibility
As I prepare for life after Concordia, I feel equipped not just with knowledge, but with purpose. I’ve learned how to ask better questions, how to sit with uncertainty, and how to seek understanding across boundaries. Neurochemistry, with its blend of scientific inquiry and human relevance, has reaffirmed my desire to work with sick children and their families – not just to read, but to listen, to advocate, and to empower.
If there’s one thing this course and this college have taught me, it’s that science isn’t just about answers – it’s about relationships. Between neurons, yes, but also between people, ideas, and communities. To be responsibly engaged in the world mean to hold those relationships with care.
So, thank you, for creating a course and a college that challenged me to think deeper, care harder, and learn more fully. I don’t have all the answers, but I get to leave with the confidence and curiosity to keep asking the right questions.
References
[1] Roberts, M. (2019, October 17). Direct support professional (DSP) job description: Salary, Skills, & More. LiveAbout. https://www.liveabout.com/government-job-profile-direct-support-professional-1669627
[2] How to become a child life specialist. Maryville University Online. (2023, October 27). https://online.maryville.edu/online-bachelors-degrees/human-development-and-family-studies/careers/how-to-become-child-life-specialist/
[3] Sensory stories by Nicole. Facebook. (n.d.). https://www.facebook.com/NicoleFilipponeAuthor/posts/because-adhd-anxiety-and-autism-often-overlap-this-is-still-one-of-my-favorite-g/147940607130063/
[4] Ed.D., J. (2019, January 27). All lessons should be interdisciplinary. Medium. https://medium.com/@jackiegerstein/all-lessons-should-be-interdisciplinary-ef619be0a747