Are helmets designed to stop concussions? I think you would be surprised to find out that helmets are not designed specifically to reduce concussion risk. Helmets are actually only tested for their ability to stop skull fracture. In the paper that was read for this week, the neurometabolic cascade of concussions was discussed. We particularly discussed the effects of concussions on the still developing brains of adolescents. Concussions are particularly bad for growing children, however, we still subject them to sports where they are most susceptible to concussions. I don’t think that taking away sports is the answer, but I do think that helmets specifically designed to reduce the risks of concussions are important. This leads to the question, how are helmets designed and tested?
NOCSAE, or the National Operating Committee on Standards for Athletic Equipment, is an organization that formed in 1969 to create helmet regulations and standards for helmet performance testing. These tests are designed to evaluate the helmet’s ability to protect against serious brain injury, but not necessarily concussions.
Recently, researchers at Virginia Tech designed their own test for evaluating helmets. This new test rates each helmet based on their ability to protect against concussions. Their results were quite shocking. The helmet used last year in the NFL, the VSR4, was second from the bottom in VT’s ratings. This is also the helmet often used by high schools and colleges around the US.
VT’s new test is a great step in the right direction for concussion protection and awareness. With this new data made public, hopefully helmet producers will focus more on safety rather than style.
Further reading
http://www.nytimes.com/2011/05/10/sports/football/10helmets.html
http://sports.espn.go.com/espn/page2/story?page=easterbrook-110719_virginia_tech_helmet_study&sportCat=nfl
In-depth information on helmet testing procedures
http://www.soimpact.com/impactdynamicsl.pdf
Justin Morneau: Wimp or Victim?
Morneaus Concussion
If you’re a rabid Twins fan like I am, you’ve probably seen this video many times. Justin Morneau’s slide in an attempt to break up a double play may have been successful, but the blow to his head resulted in a concussion just before the 2010 All Star Break in July. Voted in as the starting first baseman for the AL, he was unable to play in the game. He was unable to play the rest of the season. His level of play significantly decreased throughout the following season, and it was clear he was not the player he was a year before.
Has this concussion become a scapegoat for Morneau’s decline, while other factors are the actual contributors? Many Twins fans have displayed obvious frustration with his inability to play at his all-star capability, and some have questioned whether he is simply using the concussion as an excuse for his shoddy play. Or is it possible the concusion suffered in July 2010 was still affecting him over a year later? I am inclined to think the latter.
Before his famed baseball years, Justin Morneau played youth hockey, and has admitted to having several head injuries during that time. An article we’ve been reviewing in our Neurochemistry class talks about the mechanisms behind concussions. A large mechanical force upon the brain (such as a shortstop’s knee to your head) can cause the rapid release of the excitatory neurotransmitter glutamate. These neurotransmitters can bind to certain receptors in the brain causing potassium ions to leave the cell. In an attempt to alleviate these neurological changes, pumps in the brain work overtime to restore the brain to its normal state. This requires energy, leaving the brain in an energy crisis and causing hyperglycolisis, the break down of glucose to provide energy. Any further injury or mechanism that requires energy during this crisis could cause the brain further injury. However, this period of vulnerability can often be quite short. Pitcher Josh Beckett took a line drive to the head and experienced concussion symptoms the next day, and was cleared to pitch 8 days later. Unlike Morneau, Beckett did not have a rich history of concussions.
Why do the number of concussions have an effect on concussion symptom severity and duration? If they didn’t, Justin Morneau could have been back after a week or two. However, our article explained that repeated brain injury can result in longer lasting symptoms. After the initial period of hyperglycolysis, the brain goes into a reduced state of glucose metabolism to counter this disrepancy between energy supply and demand. The body may not be able to respond correctly to another injury during this time. Additionally, calcium ions can enter the cell and accumulate due to the receptor binding mentioned above. Accumulated calcium can lead to cellular death through various mechanisms. The brain is very vulnerable during this time. It is not necessary to receive another blow in the head for the condition to worsen; simple activity can aggravate the sensitive system. This period of vulnerability becomes longer and longer the more concussions you have, leaving the vulnerability and chance of re-injury higher for much longer. For example, Morneau returning to batting practice or fielding practice can be enough to restart concussion symptoms even without a blow or disturbance of the head.
Tests have been developed to test whether athletes are ready to return to play after a concussion. While the test is more or less a simple test of reaction time and motor ability, slight deviations from the athlete’s baseline (how well they performed on the test when healthy) may result in being held from play longer to ensure full recovery. As recent as September 2011, Morneau still did not pass this test, despite being closer than he has been over the past year. This indicates he is still suffering from some symptoms over 14 months later. Further, steps are being made in the MLB to prevent players from rushing back to play after head injuries. A 7-day disabled list was added this last season so teams could fill a roster spot during the player’s injury, making them less likely to push their athletes into returning before their full recovery.
Though the brain is able to show plasticity and resistance/recovery from injury, it is the most important area of our body to protect. Too much traumatic brain injury can lead to long lasting loss of function and even neurological diseases. It will be interesting to see whether Morneau will ever be able to recover from his latest concussion. How many concussions can you have before your brain simply can’t restore itself to normal function? With Morneau, only time will tell.
Opioid Withdrawal. . . Not a Fun Process.
This week, our class focused on an article that discussed the role of glutamate (an important neurotransmitter) in drug addiction. It emphasized opioid addiction and its effects on the brain. Abused opioid drugs include morphine, heroin, oxycodone, hydromorphone, and more. It is thought that 9% of the population may abuse opiates sometime during their life. The drugs can create physical dependence, tolerance, and withdrawal once the drug is removed. People who were given opioids in the hospital even exhibit withdrawal. Early symptoms of withdrawal include agitation, anxiety, muscle aches, insomnia, and sweating. Later symptoms include abdominal cramping, diarrhea, nausea, and vomiting. The symptoms are not life-threatening, but are uncomfortable and may encourage return to drug usage.
Withdrawal Treatments
Withdrawal treatments involve both support and medications. Some of the medications are used to treat symptoms individually. Clondidine, one of the most common medication, reduces anxiety, agitation, muscle aches, sweating, runny nose and cramping. Buprenorphine is an efficient medication that can also decease detox time. Methadone is another one that is used. However, methadone requires long-term administration with a decrease in dosage over time. Check out this website for more information and links to other aspects of drug addiction:
http://www.nlm.nih.gov/medlineplus/ency/article/000949.htm.
MK-801 and Naloxone
MK-801 and naloxone are common drugs used during opioid research. On one hand, MK-801 blocks withdrawal symptoms. On the other, naloxone is used to induce withdrawal. Both of these drugs were used in various studies cited in the paper. You might think that MK-801 has potential as a withdrawal treatment as it blocks the withdrawal symptoms. But its mechanism of action brings in some interesting side-effects. The molecule binds to the glutamate receptor NMDA, blocking it. The receptor cannot let ions through to continue a signal. This is good, right? It stops the glutamate signalling so it cannot start dopamine release. Thus there is no reward system encouraged by the opioid drugs. There is more to the story. The site to which MK-801 binds is the same as PCP and ketamine. It exhibits some of the same side-effects as these drugs, mainly schizophrenic-like symptoms. If that wasn’t bad enough, there are also cardiovascular side-effects and formation of brain lesions. And that, ladies and gentlemen, is why an effective withdrawal symptom-blocker is not used as a treatment.
Withdrawal will stop eventually, but the desire to return to the drug will remain. The reward system driven by dopamine release is strong. Opioids have been used for centuries, first in the form as poppy seeds then morphine, then heroin. Pain relief is important, so it is just as important to understand the addictive properties of the current forms we use.
MAPK: A Mechanism of Cancer
Cancer. That one word strikes fear into the hearts of Americans. Our own bodies grow uncontrollably, destroying us. People everywhere have been affected by it, whether the tumors feed off of them or their loved ones. Cancer can develop from environmental sources or genetically. Whatever the cause, malignant cells have the same characteristic: they divide and grow unrestrained. One pathway plays a key role in the development. This is the MAPK pathway, specifically starting with the protein Ras, leading to Erk. In addition to cancer, the Ras/MAPK and other MAPK pathways are also associated with Alzheimer’s disease, Parkinson’s disease, and Amyotrophic lateral sclerosis (Lou Gehrig’s disease).
Ras/MAPK Pathway in Brief
Ras is a protein that is activated by signals such as growth factors. Once activated, it can activate (which usually means phosphorylate) other proteins. For this blog post, I will focus on Ras activating Raf. Raf then activates MEK which then activates ERK. This protein will then activate several transcription factors (TF), which are molecules that encourage the turning of DNA into RNA (which can then be used to make proteins!). The majority of the TFs that ERK activates are those that encourage cellular division, growth, and migration. They also play a role in regulating cell death. With its signalling effects, the Ras/MAPK pathway plays a significant role in the development of cancerous tumors.
Role of Ras and Raf in Cancer
The genes that Ras comes from is actually a family of genes. The family includes H-Ras, K-Ras, and N-Ras. K-Ras has been found to be mutated in many types of human cancers. About 50% of colon cancer case displayed a mutation in K-Ras. Mutations in the protein right after Ras, Raf, are also present in cancer. B-Raf mutations are responsible for about 66% of melanomas, which are cancers in the skin. One of the most common mutations results in B-Raf being activated constantly. It then continuously activates the MEK. MEK’s action continues, which means MEK, ERK, and the TFs are activated as well, resulting in cellular growth that cannot be turned off.
Kind of scary, but there’s hope!
Malignant tumors exhibit uncontrolled cell growth and migration that results from genetic mutations. These mutations may have been inherited or caused by the environment. There are other oncogenes (genes that cause cancer) that are important in the development of cancer such as Rb and Myc. As we learn more about the mechanisms of cancer, we will be able to develop treatments to prevent unrestrained cell growth. A previous blog post discussed different treatment options that take advantage of our current knowledge.
Discovering Ambrosia
Extending one’s life has fascinated people for centuries. Eternal life appears consistently in mythology and religion: as ambrosia, a gift from the gods or a philosopher’s stone. Today, the average human lifespan is around 80 years. However, we are finding that more and more age-related illness, such as Alzheimer’s Disease and Parkinson’s Disease, along with cancers, are exhibited more frequently. Both of my grandmothers have age-related diseases (Alzheimer’s and PSP). The subject strikes at home for me. As such, the paper we discussed in class for Neurochemistry sparked my interest. It reviewed known mechanisms of aging and how a certain receptor, IGF1-R, may play an important role in aging of the brain and Alzheimer’s disease.
IGF1-R, Alzheimer’s, Ceramide, oh my!
IGF1-R stands for insulin-like growth factor 1 receptor. This is considered a tyrosine kinase receptor, one of the types of receptors found in the brain. These kinds of receptors usually bind to growth factors like insulin. The receptors, after binding to signal molecules, or ligands, can start a chain reaction of other signals within the cell. The signal pathways that IGF1-R activates are the PKB/Akt or Ras/MEK/ERK pathways, both important in cell growth and reproduction. The Akt pathway also encourages cell death. This plays a role in aging.
The signal from IGF1-R is thought to contribute to the switch in the presence of two different receptors: TrkA and p75 neurotrophin receptors. TrkA is exhibited more in younger animals, while p75 is shown in greater amounts in older animals. Somehow, the expression of these receptors switches; the mechanism has yet to be discovered. But it is this switch that is thought to contribute to the aging of cells and eventual development of Alzheimer’s.
p75 receptor signaling is associated with the production of ceramide, a lipid (fat). This lipid stabilizes the enzyme that breaks up the precursor molecule to the amyloid-beta. Amyloid-beta is a molecule that builds up to form the plaques that are characteristic of Alzheimer’s.
So, let’s review! IGF1-R can help cell growth, but also encourages cellular death. Somehow, it can lead the switch to p75, which will result in more ceramide. More ceramide means more enzyme; more enzyme leads to more plaques. The mechanism for Alzheimer’s is more complicated, and becomes more so everyday.
The Practical Applications of Roundworms
One thing that caught my attention is how we discovered the possible aging pathway. It was first found in roundworms. Mutations in the gene age-1 extended the roundworms’ lifespans. This gene in the worms codes for the protein PI3K which is also found in humans. Then IGF1-R was implicated as important to aging when mutations that inactivated the gene product in the daf-2 gene resulted in a doubling of the maximum lifespan of the worms. daf-2 codes for the insulin/IGF1 receptor. These genes and proteins are essentially the same as those found in humans. Further research into the human pathway confirmed that IGF1-R affects those pathways. It is logical to conclude that human lifespan, much like the roundworm’s, could be extended with mutations in the genes that code for IGF1-R and PI3K. However, we would not be able to see results right away like in the roundworm.
The fact that this information was obtained from a roundworm, something that is completely different from humans, is amazing. A simple organism can represent the “more highly evolved” organisms. All cells are connected at their roots. So next time you hear about research on roundworms, seahorses, or yeast, and think money and resources are being wasted, consider our continuing quest for life. Research is a necessity if we want to find our own ambrosia.
Tough Questions with Science: All the small things.
This week’s assignment is to explain why the public should care. Specifically, why they should care about the article we read which covers Alzheimer’s disease, Parkinson’s disease, Lou Gehrig’s disease, and Cancer. This sort of question sets off my teacher training. When setting up a lesson, teachers are challenged to give a rational on why a student should care about that issue. But, I don’t think there is much purpose in arguing the importance of specific diseases without first arguing why should the public care about the neurochemistry behind a disease.
As I trudge further into my schooling I am often reminded of the phrase “Innocence is bliss”. As a youth I would find myself in the doctor’s office for some kind of minor issue. Usually these sessions would consist of a Q & A session followed by the prescribing of some drug. To me it almost seemed implied that this one drug was the best and only solution to solve my problem. This theory was put to the test when I was prescribed drugs for my acne, (which on its own I find ridiculous).  At the time I didn’t even care enough to want to fix it. And, as if it had no consequence, I was quickly prescribed an oral drug. I was now putting medicine into my body to solve a problem that wasn’t really a problem. Here comes the interesting part. The positive effects on my acne were negligible, but after about 14 days on the drug I started experiencing horrific stomach pains. These pains left me unable to eat food for almost 24 hours, all just to “cure” a little acne. Looking back it seems quite foolish to introduce my one and only body to some biologically foreign chemical to solve something so small.
At the time I didn’t even care enough to want to fix it. And, as if it had no consequence, I was quickly prescribed an oral drug. I was now putting medicine into my body to solve a problem that wasn’t really a problem. Here comes the interesting part. The positive effects on my acne were negligible, but after about 14 days on the drug I started experiencing horrific stomach pains. These pains left me unable to eat food for almost 24 hours, all just to “cure” a little acne. Looking back it seems quite foolish to introduce my one and only body to some biologically foreign chemical to solve something so small.
This experience leads me to add a bit to the previous phrase, now I would say “Innocence is bliss, yet the informed make the best decisions”. Now don’t get me wrong, I love the idea of having doctors. I am comforted by the fact that I am surrounded by many experts in the human body whose job it is to protect my health. Yet nobody knows the exact workings of the body, everyday there are new discoveries to how we function. In neurochemistry, the focus is on molecular pathways. A chemical pathway is sort of a domino effect, one molecule interacts with another which interacts with another….. and so on.

This domino effect has pros and cons to the medical world. Think of the last domino being the one that triggers the disease. This means that there are multiple places that medicine can intervene before that final domino is toppled. The same goes for molecular pathways. Many times a medicine is targeted at a specific point in the pathway to stop the disease from being triggered. Sadly, the con must come in. It is my regret to inform you this example is an oversimplification. Fixing our molecular pathways isn’t as easy as reaching in and removing a “domino”, Instead some sort of chemical reaction must take place in your body, usually by chemicals inside of pills. Many times the problem is that these chemicals don’t only react with the “trouble pathways” but they also react with “normal pathways” and this is where side effects come into play.
So picking the right molecule to target without disrupting other pathways can be a complicated thing. From what we know from research right now it isn’t easy/possible to determine the best way to handle a disease. So the next time a doctor hands you a pill, hopefully you will be more interested in finding out exactly what you are putting into your one and only body.
The Path to Cell Destruction
This week my classmates and I dissected an article pertaining to the MAPK signaling cascade and its role in neurodegenerative diseases. Diseases such as Parkinson’s, Alzheimer’s and Lou Gehrig’s disease (also known as ALS) are characterized by progressive motor cell death impairing motor function. We always ask ourselves what the underlying cause of such ailments are, and thanks to the evolution of research in neuroscience such causes can be illuminated and eventually comprehended.
Inside of a cell there is a nucleus and it is composed of proteins that encode specific functions for the cell. The MAPK pathway is a series of chemical messengers that are activated by stimuli outside of the cell. This pathway is important for carrying out essential function such as making new/different cells and the act of executing certain cells. The MAPK pathway has many branches that activate other pathways on other organelles in the body such as the mitochondria and endoplasmic reticulum. These organelles are important for energy and making other proteins essential to the body. When mutations in the MAPK pathway occur, activity happening downstream causes a multitude of problems. Mutations in certain enzymes and proteins in the cell causes cellular function to alter and affect the functions of other organelles. The presence of free radicals cause stress on cells and the changes ensue. Constant stressing of cells and the changes that are caused by them cause components of the MAPK pathway to take a route towards cell death.
So how is the MAPK pathway associated with Parkinson, Alzheimer’s and ALS? The direct cause is not completely clear yet, but there are a lot of indications and research that supports that the MAPK pathway plays a role in these neurodegenerative diseases. In ALS, a mutation in the SOD1 gene causes oxidative stress to neurons. This mutation in the SOD1 gene makes it so a neuron cell called an astrocyte unable to clear away an area of highly concentrated neurotoxins. The high concentration of neurotoxicity causes the cell to undergo a programmed cell death. Similar activity is seen in Parkinson’s and Alzheimer’s where neurons undergo programmed cell death. The problem with these diseases is that motor function is being compromised because of neurotoxicity. Aging shows strong correlation with the onset of these diseases but are being seen as early as 20-30 year old individuals. The Concordia Cobbers are using neuroscience in collaboration with neurochemistry to study such topics so that one day we will be the pioneers of present and new research.
Link between Parkinson's, Alzheimer's, Free radicals and Anti-oxidants!
Did you know that Parkinson’s disease and Alzheimer’s disease share the same causal cellular pathway? This pathway is the MAPK pathway which is involved in cell proliferation, differentiation, survival, death, and transformation. The pathway is believed to be started when the cell is under oxidative stress. Oxidative stress is caused by imbalance between the production of reactive oxygen, which are toxic to the body, and the natural ability of the body to react with or bind to them and detoxify the free oxygen radicals. These free oxygen molecules can bind to other molecules in the cells and cause damage. Once the damage was done in one cell, the free oxygen can spread to neighboring cells and cause damage to them as well.
Here is a brief explanation of how these two diseases share a common activation pathway. When a cell is under stressful condition due to, for example, genetic mutations or aging, the free radical formation is increased and this activates the MAPK pathway, specifically those activate p38 and JNK, which facilitates the mechanisms of cell inflammation and eventual cell death. In the Alzheimer’s disease and Parkinson’s disease, the MAPK activation leads to the death of the neurons which release dopamine, an essential neurotransmitter to mediate cellular activities in the regions of the brain important for memory, movement, and several other physiological and psychological functions.
As we talked about Alzheimer’s disease in my blog from last week, let’s take a brief look at Parkinson’s disease and MAPK pathway this week. Parkinson’s disease is mostly characterized by the formation of Lewy bodies in Substantia nigra of the brain. Substantia nigra is the region in the brain that is rich in dopamine releasing neurons called ‘dopaminergic’ neurons. These dopaminergic neurons project to Nigrostriatal pathway in the brain which is involved in controlling movement.
Normal neurons of Substantia Nigra

The main content of these Lewy bodies is the protein called Alpha-synuclein which is normally present in the synapses and nuclei of the nerve cells. The over-expression of the gene that encodes for Alpha-synuclein is usually caused by genetic mutation or oxidative stress which blocks the active degradation of this precursor gene. This over-expression leads to abnormal aggregation and deposition of Lewy bodies in the dopaminergic neurons of Sustantia nigra. As a result of this aggregation, these neurons are exposed to inflammatory reactions and eventual cell death and hence cause dysfunctions in mediating movement.
Lewy bodies in the neurons of Substantia Nigra of Parkinson’s patient

One of the most natural solutions to prevent and reduce these neuronal abnormalities is the anti-oxidants. Antioxidants are the compounds that can bind to the free radicals and detoxify them by giving an electron to free oxygen molecules. Antioxidants are naturally present in many fruits and vegetables that are rich sources of Vitamin C, E, Flavonoids and Carotenoids. Researchers have confirmed the positive effect of antioxidants in preventing aging related diseases like Alzheimer’s, Diabetes, and neurodegenerative diseases such as Parkinson’s disease.

It is recommended to have healthy diet of fruits and vegetables such as, grapes, citrus fruits, grapefruit,etc., rather than taking anti-oxidant supplements as the overdose of antioxidants can interfere with normal physiological functions of the reactive oxygen. Hence, it is important to have a healthy diet containing fruits and vegetables and to keep the optimum amount of anti-oxidants in the body in order to slow down aging and related diseases and to prevent other dozens of diseases such as cancer which are induced by oxidative stress.

Breaking Bad's Cancer

I’m sure many of you have at least heard of AMC’s hit show Breaking Bad. The show follows the story of Walter White, a high school chemistry teacher who was diagnosed with terminal lung cancer. In order to leave his family in a financially stable state when he dies, Walt starts synthesizing methamphetamine. Later on in the show, he starts his chemotherapy treatment. So, what exactly is chemotherapy?
Chemotherapy, generally, is a term for a treatment of any physical ailment with chemicals or drugs. Now, however, the term is almost always used to describe cancer treatment specifically. Before we can outline and understand the treatment given to Walt, we need to understand lung cancer. Lung cancer is just that, cancer that starts in the lungs. It is an uncontrolled cellular growth in the lungs. There is a mishap in the cellular signaling that sends the cells on a division spree. This is caused by a mutation in epidermal growth factor receptors (EGFR). These receptors regulate the cell cycle, meaning it controls division, proliferation, and apoptosis of the cell. It can also be caused by mutations in the p53, tumor suppressor gene found on chromosome 17. (A great paper on lung cancer progression can be found here)
Now back to the question before, what is chemotherapy in the context of lung cancer?
The most common drugs used in treatment are cisplatin and carboplatin. Cisplatin works in a very interesting way. It crosses into the nucleus and interferes with DNA. It prefers to bond to guanine out of the bases. By binding to the guanine, it can cause crosslinking in DNA. This calls in DNA repair mechanisms, which in turn causes apoptosis to occur. Pretty cool stuff.
Carboplatin works in much the same way, but it just has a different chemical structure than cisplatin. It also has reduced side effects. Nausea and vomiting are not as severe and are easily controlled. It is also less toxic to the kidneys.
So, there you have it, now you can understand a little bit of what exactly Walt is going through when he receives his treatment. He may be on cisplatin with the way he throws up so often, but we won’t know for sure unless the show tells us! Thanks for reading.
Stress, the Silent Killer
For years, doctors and other health professionals have been telling us that too much stress can kill us.
Well, it’s true.
Increasing research is showing that stress in the body changes how cells, systems, and organs function. Too much stress in our bodies can lead to increased cell death and many different kinds of diseases.
What kind of stress are we talking about?
Free radicals are chemicals that contain oxygen and are unstable, highly reactive substances. When the body uses and breaks down oxygen from its environment, free radicals are formed. These molecules are important for many cellular processes. But when levels of free radicals rise past a point, the excess amounts can have harmful, stress-producing effects on the body. This is called oxidative stress—stress at the cellular level that changes physiological functioning.
This can seem like an apparent contradiction: too much oxygen (obviously necessary for life!) can be toxic? However, it is a natural by-product of respiration and metabolism. Regular oxygen intake, as well as increased levels from exercising, must be metabolized by the body, which results in the formation of free radicals. We also encounter oxidative stress in the environment from heat, UV radiation, pollution, and a host of other chemicals we encounter frequently. These sources of free radicals alter physical functioning, contributing to many diseases.
The effects of oxidative stress
The map kinase (MAPK) pathway in the body is a series of biochemical reactions that are vitally important for many physical processes, including metabolism, growth, the production of new cells, and the disposal of old, damaged cells. This critical pathway has been the subject of our class readings and discussions this week as we have investigated how the MAPK pathway is involved in diseases like Alzheimer’s, Parkinson’s, Lou Gehrig’s (ALS), and the big killer, cancer. Something all these diseases seem to have in common is oxidative stress as a factor negatively affecting the function of the MAPK pathway.
How exactly does oxidative stress alter this important biological pathway? Stress can overactivate microglia, cells in the brain responsible for repairing damage. If there is too much microglia activity, they “clean up” tissue a little too much, killing and removing more cells than is necessary. This may be what is going on in Alzheimer’s Disease, a degenerative disease characterized by loss of brain tissue.
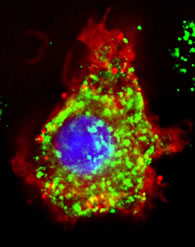
Microglia (green) busy at work!
Oxidative stress can also change the states of genes that code for important proteins and factors, including a family of proteins called ubiquitins that break down and recycle old proteins. If these proteins aren’t functioning correctly, other kinds of proteins can build up into significant deposits in tissue, leading to impaired function. This is evident in both Alzheimer’s and Parkinson’s Disease, both of which show pathological amounts of proteins building up in the brain.

Plaques (orange circles) caused by protein deposits in the Alzheimer’s brain
SOD1 is an enzyme that converts harmful free radicals into less dangerous substances. However, it can be de-activated by too much oxidative stress, allowing free radicals to build up and areas of the body to become inflammed. In both Lou Gehrig’s Disease and Parkinson’s Disease, inflammation of the nerve cells may contribute to the loss of neurons, a primary characteristic of these diseases.
Dopamine neurons, lost in Parkinson’s Disease, are critically important for movement abilities
Finally, the MAPK pathway is necessary for controlling the cycle of cell death and growth, which allows for new cells to replace old ones. However, in cases like cancer, if this pathway is harmed, cells can multiply rapidly, forming tumors. In other cases, cells die too quickly and are not replaced right away. Disrupting this pathway through oxidative stress can have a severe effect on the body.
How is oxidative stress combated?
Antioxidants! These molecules can come from a variety of sources and effectively neutralize free radicals in the body. By breaking down free radicals, they have the potential to stop the damage of oxidative stress. Research studies are currently investigating how antioxidants can protect the body from damage as well as help treat diseases like Alzheimer’s and Parkinson’s.
Some kinds of berries, like blueberries, contain high levels of antioxidants
Some sources of antioxidants include vitamins C, E, A, and polyphenols, to name a few. These are commonly found in many fruits, dark green or yellow vegetables, oils, tea, and wine. Other natural antioxidants like melatonin and ubiquitol are produced in the body already. As the value of these substances continues to be demonstrated by scientific research, they may become a useful nutritional therapy for degenerative diseases like Alzheimer’s, Parkinson’s, Lou Gehrig’s, and cancer.
