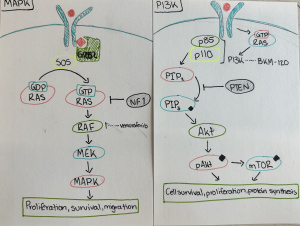
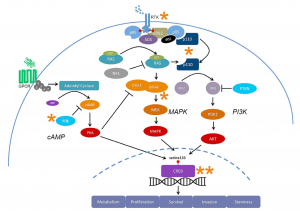
This semester has been nothing short of transformative. As I look back on my experience in the neurochemistry capstone course, I realize how much it has helped me grow—not just as a student, but as a thinker, a future health professional, and a more reflective person. When I enrolled in the class, I expected to learn about brain chemistry, neurotransmitters, and perhaps a few disorders. I didn’t expect that the discussions, the research, and the questions we asked would lead me to reimagine my relationship with learning, my goals for the future, and my role in the world around me.
One of the most eye-opening moments of the semester came when we studied metabolic syndrome. This paper wasn’t just scientific—it was deeply personal. I began to see how diet and lifestyle could influence not only physical health, but brain function and emotional regulation. For the first time, I started asking myself questions I had never considered before. What am I feeding my brain? How do my daily choices impact my long-term well-being? Learning about the biochemical effects of diet on cognition challenged me to reflect on how I could live a more fulfilling, sustainable life. It wasn’t just about the science—it was about applying that science to my life in meaningful ways.
That connection between the brain and the world kept coming up. Every topic we explored, from dopamine pathways to the role of inflammation in behavior, circled back to a larger truth: that we are all shaped by more than just biology. Our environment, our culture, and our experiences all play roles in the chemical makeup of our minds. This realization made me even more passionate about pursuing public health as my next step. I want to take what I’ve learned and give it back to the communities that raised me, by educating people about how their health is tied to both internal and external systems—especially those who might not have access to this kind of information. This class helped me see that science isn’t just about the lab or the textbook. It’s about people. It’s about lives.
Throughout the semester, I found myself developing skills that I know will stay with me far beyond college. I learned how to analyze dense research articles, interpret complex data, and make sense of experimental design. These skills helped me see the world differently. Now, when I come across a chart or a headline, I pause to ask deeper questions. What’s the source? What are the variables? What story is the data trying to tell? These habits have made me more critical, more thoughtful, and more curious. If I were to highlight one skill on my resume that I’ve strengthened the most, it would definitely be data analysis. The ability to break down research and extract meaningful insights is something I now feel confident doing—and something I’m excited to continue refining in graduate school.
What makes this class so memorable isn’t just the knowledge I gained, but the way it encouraged me to think across disciplines. Though it was grounded in neurochemistry, the course constantly pulled in ideas from psychology, nutrition, sociology, and even philosophy. We didn’t just learn about molecules—we asked what they meant for real people in real situations. This interdisciplinary approach helped me see problems from multiple perspectives. One example that stands out was when we discussed the biochemical effects of trauma. Instead of stopping at cortisol and amygdala function, we talked about how poverty, systemic racism, and lack of access to resources can trigger those same pathways. That kind of thinking—holistic, interconnected, and human—is the kind of thinking I want to bring into my work in public health.
More than anything, this class helped me understand myself. When we read about stress, addiction, and neurological imbalances, I sometimes saw my own experiences mirrored in the science. I thought about family members, about friends, about people in my community who live with challenges that are often dismissed or misunderstood. This sparked a sense of empathy and purpose in me that I hadn’t expected. It made me want to do more than just understand the brain. It made me want to use that understanding to advocate, to educate, and to care.
Being at a liberal arts college has played a huge role in shaping this mindset. I’ve come to value the freedom to explore, to ask questions, and to draw connections across subjects. The environment at Concordia has helped me see that learning doesn’t have to be rigid or one-dimensional. It can be dynamic, creative, and deeply personal. It has shown me that education is not just about knowledge, but about transformation. It is about becoming someone who engages thoughtfully with the world.
One of the most valuable things I’ve taken from this class is the ability to solve problems by drawing from multiple disciplines. When I’m faced with a challenge now, I no longer look for a single answer from a single field. I ask how biology, psychology, and social structures might all be playing a role. I look for intersections. That mindset is something I’ll carry with me into every future classroom, clinic, and community I serve.
This class didn’t just teach me about neurotransmitters. It taught me about people, purpose, and possibility. It reminded me that the brain is not just a network of neurons—it is a reflection of the environments we live in and the lives we lead. And most importantly, it reminded me that I have the tools to make a difference. That is what learning at Concordia has given me. And that is what I hope to pass on.
I also want to give a special thank you to Professor Mach for making this year a great one!

:max_bytes(150000):strip_icc()/government-job-profile-direct-support-professional-1669627-final-24f90e7cdcb841c3b2954fa4997613dd.png)