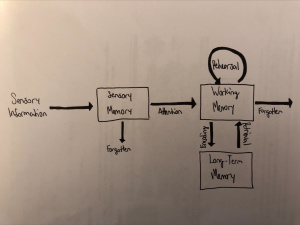
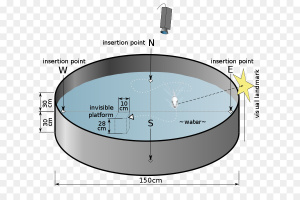
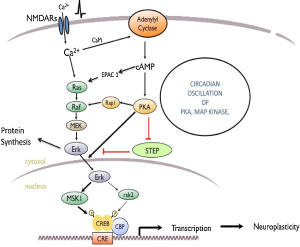
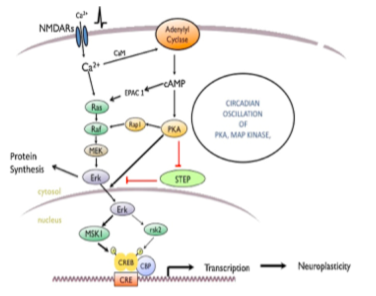
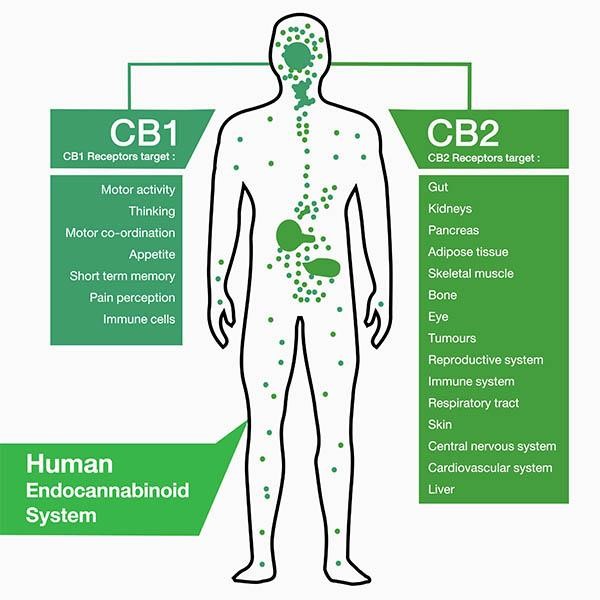
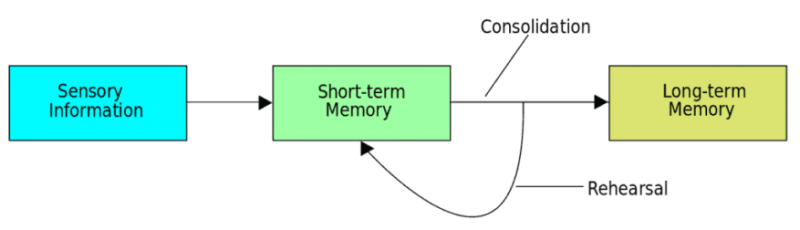
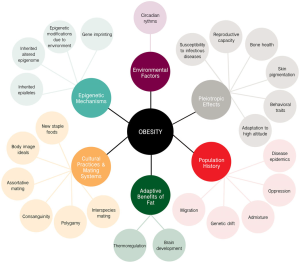
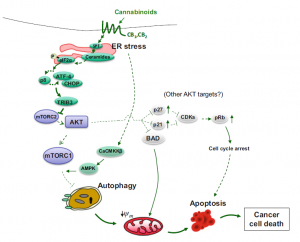
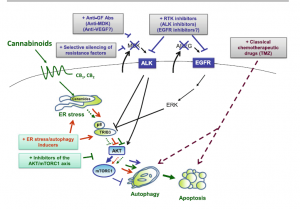
I found that this specified class was unique compared to the other ones that I have taken during my time at Concordia. However, it was a good experience that helped me be able to understand more on comprehending data and information on multiple scientific articles. From what I learned from this class, I believe that visual and audio learning, from reading, are important types of learning for me when trying to comprehend complex scientific writing. The visual imagery of each neural pathway helped me understand what exactly goes on in the brain, allowing myself to understand the scientific writing. Hearing myself talk and converse on the topic also helped me as well.
I found myself learning more on how to understand scientific writing styles and the skills, competences, and knowledge gained from this experience allowed me to apply this mode of study to other assignments and projects. An example in this instance is this senior seminar paper I am writing on solutions to climate change. The papers I read for my review are scientific articles and applying from what I learned on reading these types of articles in neurochemistry, I am able to comprehend what goes on in each of the experiments conducted in each paper. While this cannot be considered as a future goal right now, it was in November when I was writing my first draft for my review.
Along with applying this way of study to my assignments, it has helped me understand more on the concepts of vocational value and liberal arts. Learning at a liberal arts institution to me is learning how to create a strong sense of social responsibility and expand your vocational values, expanding your intellectual and practical skills, such as communication, analytical, and problem-solving abilities, and a demonstrated ability to apply knowledge and skills in real-world settings. These new skills that I have obtained can be highlighted on my resume as skills and competencies that prove that I have improved by way of thinking on a problem and answer it with the best answer possible. An example of a type of problem I solved was describing how two neural receptors are similar but have different functions when a mechanism is carried out. Reading carefully and efficiently through this part of the paper is key.
The skills I believe that I have improved very well are reading comprehension, statistical analysis, and visual learning through studying certain parts of each research paper, or article. This includes scientific functions of each neural pathway written in the paper, understanding the results of each experiment, and once again the ability to learn by seeing the example of the neural pathway present in the paper. Overall this type of class has shown me not just how to apply ways of study to a paper and understand it, but also to understand more on how each disease is caused and how certain mechanisms that happen in the brain develop. These were very interesting articles to read and I found that the course information was very interesting and fun to learn about.