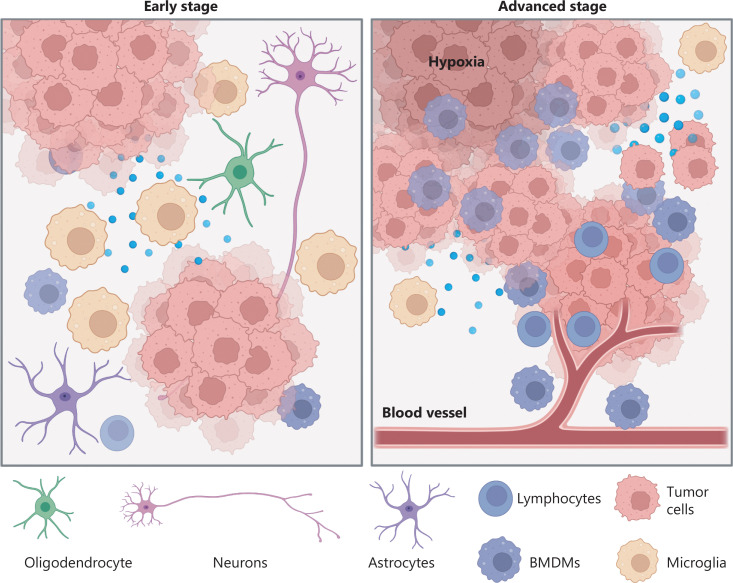
What makes a college experience beneficial beyond the gaining of a degree and/or the making of college, sometimes lifetime, friends? Concordia College, and other colleges or universities, may argue that it is the instillment of liberal learning within a curriculum, which is abstractly illustrated in Figure 1.
What is Liberal Learning at Concordia College?

Figure 1. Creatively illustrates the multiple aspects that go into creating a liberal learning curriculum.1
The five goals for liberal learning at Concordia are:
- To instill a love of learning
- To develop foundational skills and transferable intellectual capacities
- To develop an understanding of disciplinary, interdisciplinary and intercultural perspectives and their connection
- To cultivate an examined cultural, ethical, physical and spiritual self-understanding
- To encourage responsible participation in the world1
To learn more about the core curriculum of Concordia, click [here]2. To analyze whether these goals have been achieved, I’ll be reflecting on my experience taking Neurochemistry (Neu) 475 and analyzing if it proved beneficial to my overall college experience.
Learning
I came into college with “unlabeled” love for learning. I say “unlabeled” because I never outrightly thought that I loved learning; I just knew that I was driven to do well in classes and would sacrifice social time to understand a concept fully. But once I got through more difficult classes and realized the commitment they’d require to do well in, I realized my deep desire to learn. Since then, that desire has been instilled even deeper, and I have grown to love learning so much at Concordia, to the point where I still try 100% in my assignments and exams despite being a senior experiencing senioritis.
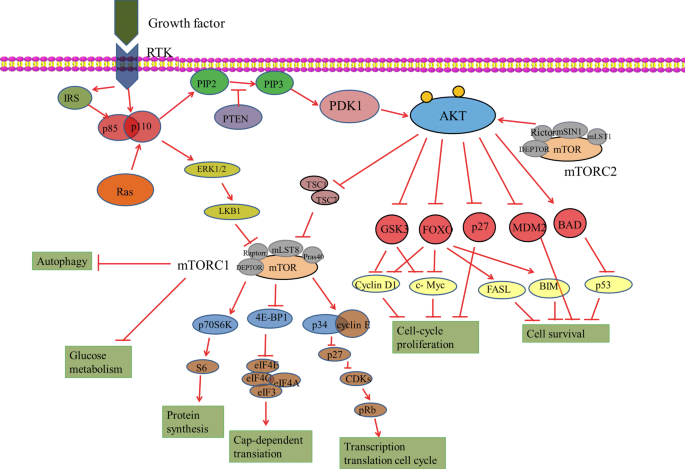
Looking at this semester in neurochemistry, I went through different types of learning. For example, when coming up with questions to ask during class regarding the papers we’d just read, I tried to analytically learn what the paper was telling me and decipher what I did not understand. Not only did this allow me to engage in analytical learning, but the reading and writing required to complete each literature worksheet forced me to perform engaged and intrapersonal learning, where I was wrestling with the text to get the main point of every section. Not only that, but the following research on our assigned topic and class discussions about our topics got me to perform interpersonal, active, aural learning where I engaged with my classmates who had background of topics different than mine. This allowed me to expand my knowledge and practice active listening, making my learning more holistic in nature. Lastly, the creation of artstracts allowed me to explore my creativity and learn how to portray a scientific concept abstractly. See Figure 2 for my artstract on schizophrenia and how the Wnt signaling pathway and BCL-9 may be involved.

Figure 2. This is an artstract from my blog post, “A Pathway to Schizophrenia”.
Foundations & Transferability
Not only that but taking Neu 475 stimulated by love of learning by allowing me to learn the foundational knowledge of the different signaling pathways used by neurons. This included learning/reviving my memory on information like the different types of receptors, types of cellular communication, excitatory and inhibitory neurotransmitters and their pathways, and different signaling pathways. This information was essential to understanding the class papers without feeling overwhelmed, so having the intellectual capacity to transfer what I learned about such information into the papers’ explanations of different irregularities leading to neural dysfunctions proved very beneficial, and it’s an ability I improved while taking Neu 475.
Disciplinary, Interdisciplinary and Intercultural Perspectives and Their Connections
Some of this information was easily transferable to other classes I have taken, specifically histology, anatomy & physiology, genetics, and biochemistry. So, while taking Neu 475 certainly increased my knowledge in the field of neuroscience, my background from other classes allowed me to appreciate the interdisciplinary impact the information had.
- For example, my background in genetics allowed me to understand more easily why activation or inhibition of different transcription factors had detrimental effects like development of brain tumors.
- In the example of histology and physiology, my knowledge on what melanocyte stimulating hormone (MSH) does in the skin caused me to be surprised when I learned in a paper that they had a role in regulating appetite regarding metabolic disease. To solve the question of what exactly MSH does and how, I gathered research from histological, physiological, and neurological sources to discover all the MSH is involved in and the pathways by which is causes such things. It also furthered my understanding regarding the different barriers scientists must pay attention to of when thinking of using a particular hormone or neurotransmitter as a target for treatment of one disease, as modifying its levels may have unintended consequences on its other bodily roles.
- This was just one example of how in Neu 475, I used disciplinary and interdisplinary perspectives to connect dots to try and solve a problem.
Regarding intercultural perspectives, talking with my classmates from different cultures expanded my knowledge regarding their points of views on certain issues. It allowed me to hear and understand the reasoning behind their opinions that differed from mine, broadening my point of view and allowing me to think of the implications certain decisions may have on others. For this reason, I am extremely grateful for the discussion days we had during Neu 475.
Cultivating an Examined Cultural, Ethical, Physical and Spiritual Self-Understanding
Along those same lines, the discussion involved in Neu 475, along with other classes I’ve taken during my time at Concordia, have caused me to examine my cultural understanding of how others are impacted differently by certain decisions and how they would prefer certain actions be taken over others. Neu 475 discussion focused a lot on the ethical implications different moves taken towards targeting different diseases would have on varying populations, with the discussions on autism spectrum disorder and addiction being the first discussions that come to mind. These discussions provided new points of views that have now been incorporated into my ethical self-understanding.
Not only that, but the reading of papers and class lectures and corresponding research grew my physical self-understanding of what is going on within my body. So overall, Neu 475 alone did quite a bit to encourage me to examine my cultural, ethical, and physical self-understanding. Because most of this was done through discussion and in-depth communication between my peers, if I were to highlight a skill I improved through this class, it would be interpersonal communication. I grew my understanding of the value of listening whilst communicating and practiced how to time and word my responses in ways that would be most well received by those I was communicating with. The preparation required to engage in these discussions and speak well with peers involved the interpersonal skills seen in Figure 3.

Figure 3. This illustrates different interpersonal skills that were involved when having discussions with my peers during Neu 475 class discussions.3
Responsible Participation in the World
As an aspiring physician, learning about all the different factors that are being considered as contributors to different disease or conditions was eye-opening. It taught me that I need to continually keep myself up to date on the research that is coming out regarding these conditions so that I can better target the root sources of conditions with the treatments I prescribe having the least number of negative impacts on the body. Neu 475 also gave me a very good background into the different mechanisms by which the brain communicates to the rest of the body, and while I know I need to learn much more to become a doctor, I know that the knowledge I have gained will only benefit me when it comes to making responsible decisions and giving sound advice as a doctor.
Not only that, but I have already been implementing the things I’ve learned in Neu 475 in the advice I give to friends regarding their diet and how it impacts their health. For example, I have told myself and my friends to eat less saturated fatty acids because it induces hypothalamic inflammation, which under long term conditions, leads to obesity because it messes with your hunger-inducing or satiety hormones, and because my friends and I are all STEM majors, we are more receptive to this advice because I back it with biological reasoning.
Conclusion
Overall, Neu 475 is a great example of a class that implemented the practice of liberal learning into its teaching style, its class activities, and the nature of the information it taught. By doing so, it greatly improved by overall college experience, and reflecting on how it has done so has allowed me to reflect more broadly on everything I have learned throughout my undergraduate education. Neu 475 successfully accomplished all the five learning goals for liberal learning as set by Concordia College, and those that it focused less on, like cultivating an examined spiritual self-understanding, were deepened through other classes taken during my time at Concordia. However, the five learning goals were also achieved in classes before Neu 475, but it was during Neu 475 that they were clearly and successfully integrated and applied. Thus, it can confidently be said that instillment of a liberal education proved beneficial to my college experience.
Learning at a liberal arts institution improved me as a person, and to me, means that I was able to holistically grow myself so that in the future, I can make decisions with the knowledge of how they may impact certain populations, even if I do not directly interact with them much. Whether this be in my prospective job as a doctor or in more broad decisions like voting yes or no on certain ballots, I know I am much more well-educated to make such decisions.
Footnotes:
2https://catalog.concordiacollege.edu/core-curriculum/core-curriculum/#text
3https://www.thebalancemoney.com/interpersonal-skills-list-2063724


 Abstract by Alisha Debleye depicting the tumorous and dangerous growth in the human brain that can sometimes go untreated or undiagnosed.
Abstract by Alisha Debleye depicting the tumorous and dangerous growth in the human brain that can sometimes go untreated or undiagnosed.