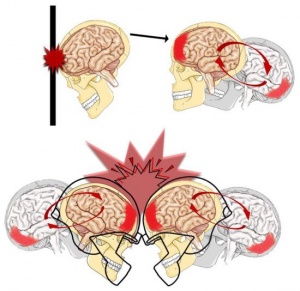
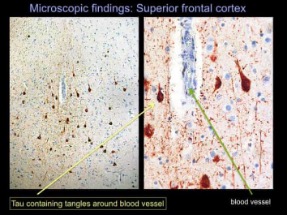
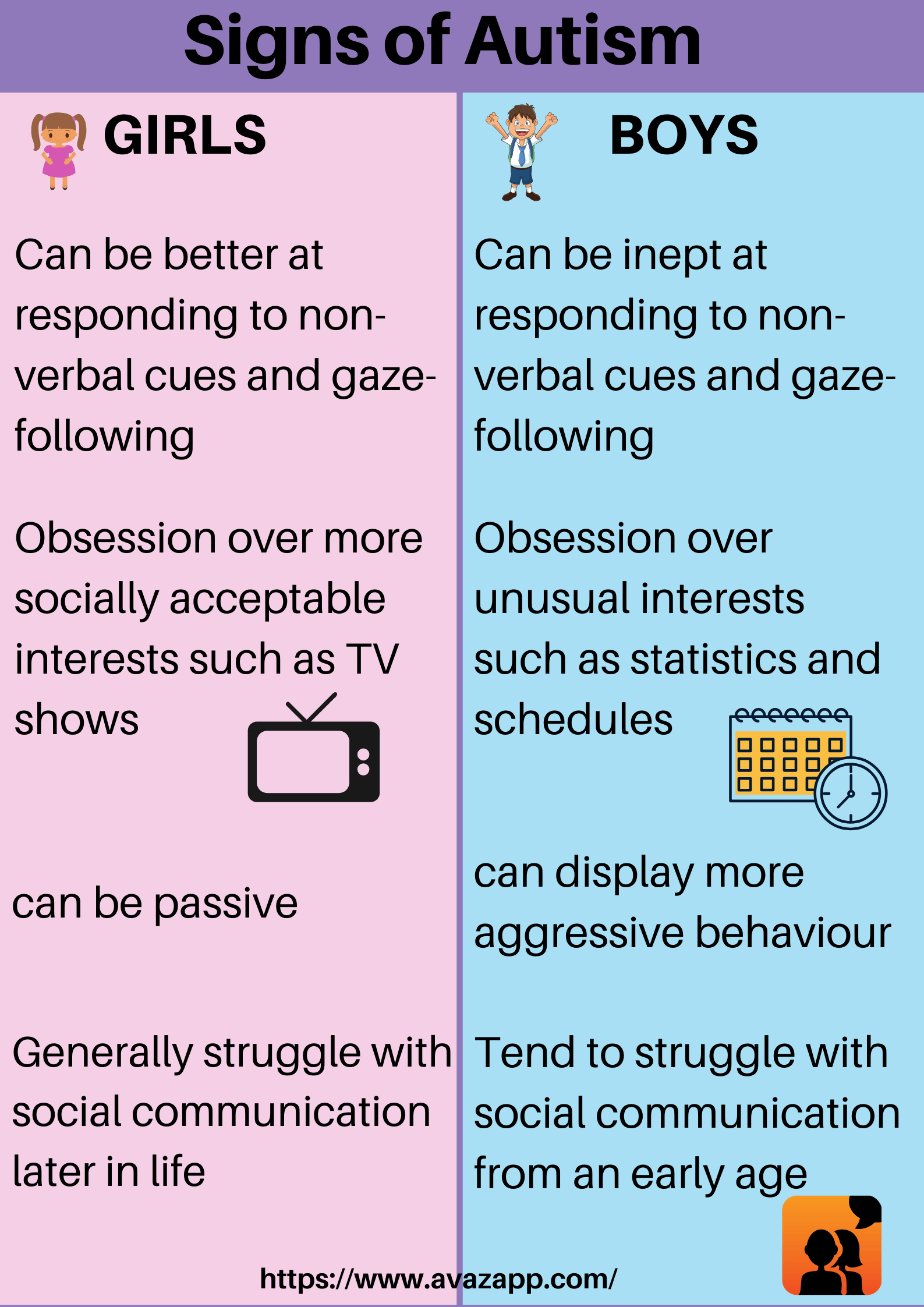
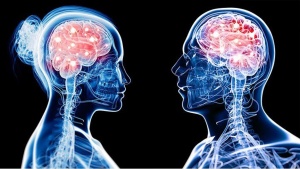
About 1.6 to 3.8 million sports and recreational concussions occur in the USA each year. 1 in 5 of these individuals will develop mental health concerns and disorders. Signs and symptoms that are severe can last as long as 6 months. Females, the elderly population, athletes, and individuals in the military are the most susceptible to concussions. Females are more susceptible because of weaker and thinner neck muscle fibers. Below is an image showing these differences: (3, 5)
Mental Health symptoms after experiencing a concussion include but are not limited to:
- social anxiety
- irritability
- anger
- depression
- feelings of overwhelm
- general anxiety
- mood swings
- emotional lability
- impatience
- difficulty concentrating
- brain fog
- sensitivity to light
- fatigue or burnout
- memory loss
- sleep problems
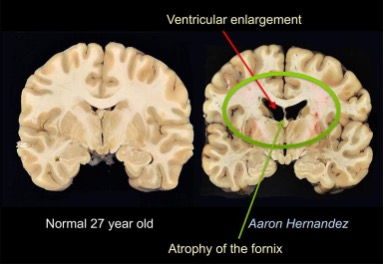
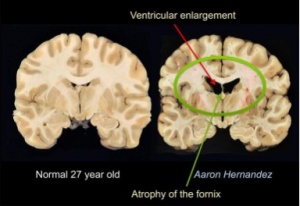
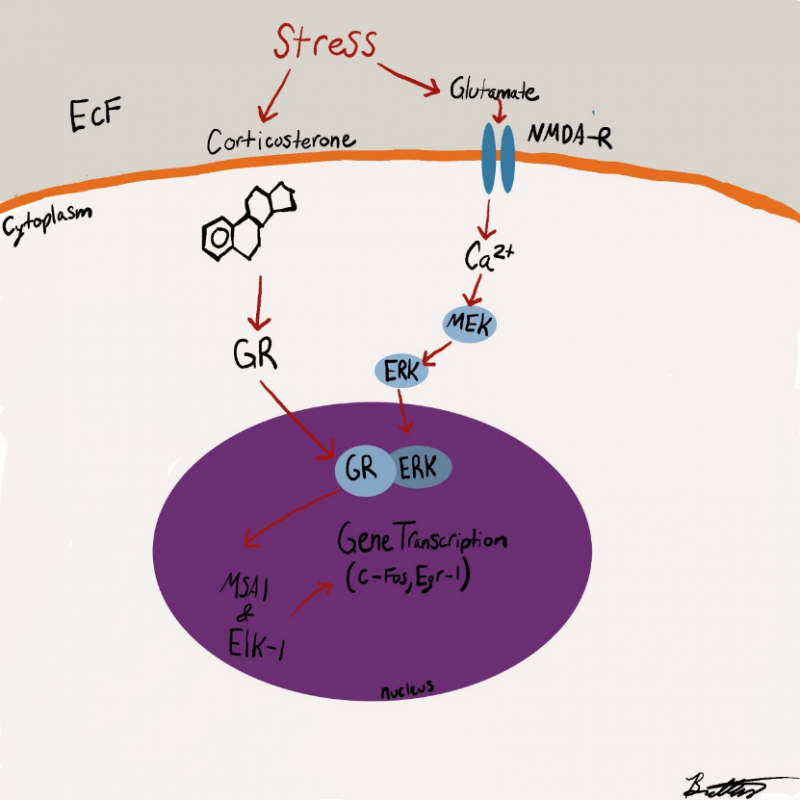
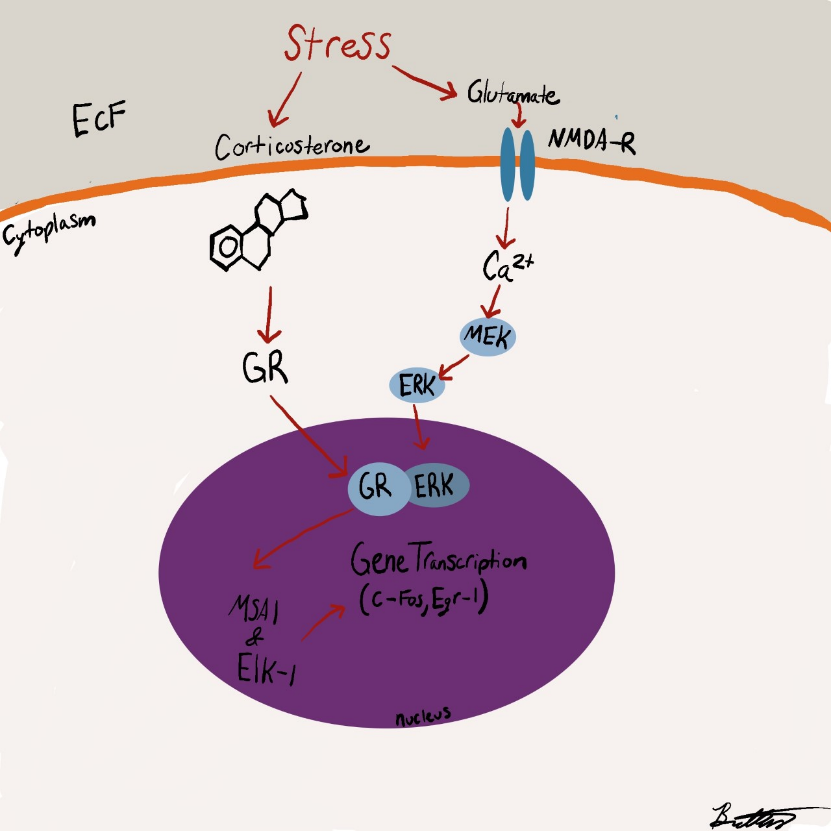
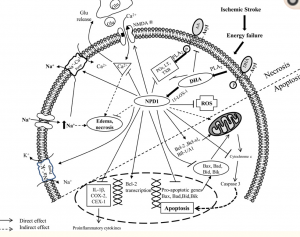
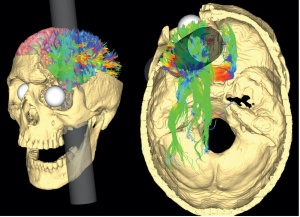
Researchers have stressed that an individual’s personality doesn’t change after a concussion or traumatic brain injury but their behavior does. Their mood can be altered due to the symptoms of a TBI such as poor quality or lack of sleep, the frustration of not remembering things, feeling burnout easily, developing anxiety, etc. Personality is described to be a part of the person such as their dislikes, favorite food, activities, color, people, etc. How the individual reacts to such variables may change but they are not necessarily gone. This is not to say that personality can’t change because it can be depending on the location of the injury such as Phineas Gage in which his personality and behavior dramatically changed after suffering an injury to his frontal lobe. The diagram below shows the impact of the incident on the damaged regions. (7, 1)
One study found that experiencing a TBI can increase your chances of developing a mental health disorder by 439%. Many people suffer from social anxiety, irritability, anger, depression, feelings of being overwhelmed, general anxiety, mood swings, or emotional lability (uncontrollable or inappropriate crying) after their injury. (3)
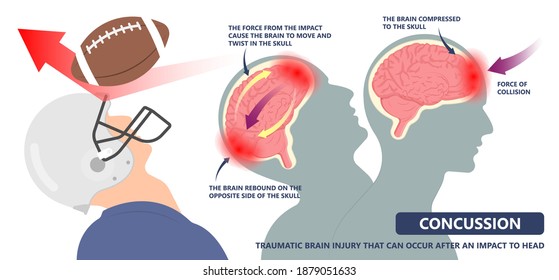
Concussions disrupt the communication between neurons and the blood vessels along with damage white matter within the brain. The damaged pathways will take a couple of weeks to heal but full recovery is expected for nonrepeated or infrequent concussions. In 30% of individuals who experienced concussions noticed a long-lasting slow and disruptive cognitive processing and responses. (1)
Females, the elderly population, and individuals who have had prior experiences of concussions, seizures, migraines, learning, mood, and anxiety disorders are the most susceptible to experiencing PCS (post-concussion syndrome). PCS is when the individual has prolonged symptoms and has continued experiencing such signs and symptoms past the expected recovery time given by doctors. The NINDS found that 20% of individuals who were in the military had experienced injuries during Iraq and Afghanistan. Of this 20%, 83% obtained a concussion or mild traumatic brain injury. Depression after sustaining a concussion is a common symptom. These individuals are twice as likely compared to individuals without TBI’s, to be at higher risk for self-harm and attempting suicide. Depression may contribute to the struggles that come with social interactions after a concussion as the person becomes easily fatigued and irritable due to intense lighting, loud noises, or too much movement and stimuli. (5)
Overall, concussions can lead to behavioral changes due to the symptoms and signs followed by the incident. Seeking professional help on how to manage these symptoms and signs is encouraged.
Resources:
- https://www.cognitivefxusa.com/blog/personality-changes-after-a-brain-injury-or-concussion
- https://www.nih.gov/news-events/news-releases/mental-health-disorders-common-following-mild-head-injury
- https://sciencenordic.com/biology-denmark-depression/head-injury-can-cause-mental-illness/1395035
- https://www.cognitivefxusa.com/blog/multiple-concussions-effects-and-treatment
- https://www.medicalnewstoday.com/articles/326227#complications
- https://www.spokesman.com/stories/2019/apr/03/researcher-mental-health-issues-often-progress-aft/
- https://www.npr.org/sections/health-shots/2017/05/21/528966102/why-brain-scientists-are-still-obsessed-with-the-curious-case-of-phineas-gage

 (Kohl’s building blocks)
(Kohl’s building blocks)
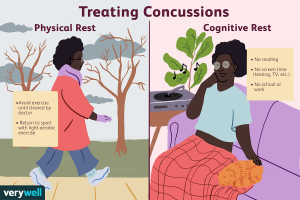
 should include the avoidance of:
should include the avoidance of: