What is addiction?
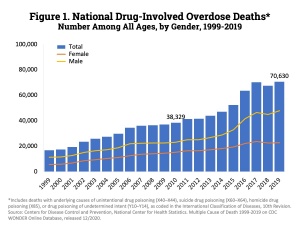
Addiction is characterized by an uncontrollable urge to engage in behaviors that are pleasure to someone, but can have extremely negative neurological impacts. In this blog, the focus will be on addiction to substance addiction, such as cocaine and opioids. Drug addictions are dangerous because illicit drugs create neurological changes that extend far beyond the high.
Neurological changes in addiction
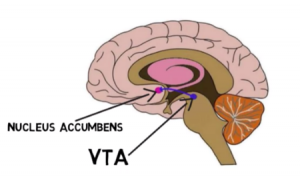
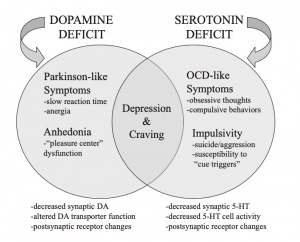
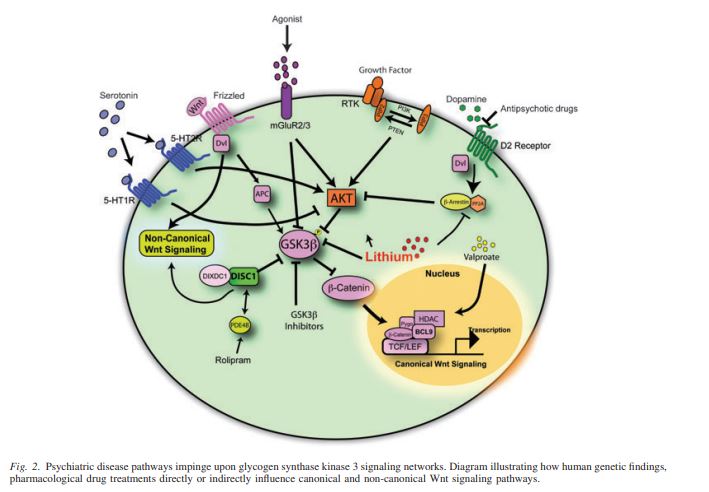
The main pathway of addiction that leads to repetitive and overwhelming behaviors to seek out drugs is influenced by the mesolimbic dopamine system, responsible for releasing dopamine from the ventral tegmental area (VTA) to the nucleus accumbens and other structures of the brain responsible for creating memories. Memory forming structures include the nucleus accumbens, hippocampus, amygdala, and prefrontal cortex, all of which aid in synaptic plasticity.
Synaptic plasticity is a neuron’s ability to strengthen or weaken the communication between neurons, which allows for the brain to learn and store memories. With drug exposure, the neurons learn and form memories through synaptic plasticity. When there is prolonged drug exposure, positive and negative behavior reinforcements are responsible for how drug-related behaviors begin.
Factors that reinforce behavior:
CREB
Negative behavior reinforcement is modulated by a transcription factor, CREB. CREB decreases pleasure from taking drugs and contributes to uncomfortable physical and emotional symptoms when the drug level decreases, contributing to tolerance and dependence with every drug exposure in the VTA and nucleus accumbens.
DeltaFosB
Positive behavior reinforcement is modulated by a transcription factor DeltaFosB. DeltaFosB is upregulated with drug exposure in the nucleus accumbens, and helps to form new synapses on dendrites to influence learned behaviors and memories related to drugs. Positive reinforcement from DeltaFosB increases the reward and pleasure sensation from taking drugs, which is seen in most, if not all, addictive drugs.
BDNF
With opioids and even marijuana, the act of taking these drugs can decrease BDNF produced in dopamine neurons. Reduced BDNF synthesis can decrease the activation of a tyrosine kinase receptor, which then reduces the activation of IRS-2, Akt, and mTor. The downregulation of this causes neurons in the VTA to lose volume. The loss of neuronal volume can increase an addict’s neuronal excitability and decrease dopamine release on the nucleus accumbens, which can increase the symptoms of tolerance and dependence seen with CREB. Stimulants, such as cocaine, increase BDNF signaling in the nucleus accumbens to strengthen the behavior to administer drugs, which is similar to DeltaFosB.
Who is at risk for addiction?
Genetics, environmental factors, and previous drug (nicotine, marijuana, cocaine, meth, etc.) exposure all pose potential risks for developing an addiction. Being surrounded by friends or in an environment with drugs can influence someone’s decision to try various substances. Adolescents who have had drug exposures can be at an increased risk compared to adults.
Youth drug exposure:
In adolescence (13 years-20 years), the brain has an increased capacity to learn and form memories compared to adults. With the increased capacity for synaptic plasticity of adolescents, exposure to gateway drugs or street drugs can create an increased ability to learn and store memories associated with that drug compared to adults. Also, as the brain is still undergoing development, adolescents experience a heightened sense of reward from dopamine compared to adults because DeltaFosB is more readily expressed in adolescents than adults. Also, adolescent brains do not experience as much negative reinforcement as adults do. Then, the prefrontal cortex, controls impulse, does not fully develop until the mid-twenties.
These factors of decreased negative reinforcement, increased positive reinforcement, and an immature prefrontal cortex means that taking drugs is more pleasureful and thinking about the full impact of taking drugs is decreased. On top of the pleasure, the synapses from learning and memory formation from taking the drugs are more extensive than adults due to increased DeltaFosB synthesis, making addiction in adolescence hard to treat. Additionally, some brain structures decrease in size, such as the amygdala and pars opercularis, which also control impulse, as seen in Figure 1.
Brain Structures and Drug Use:
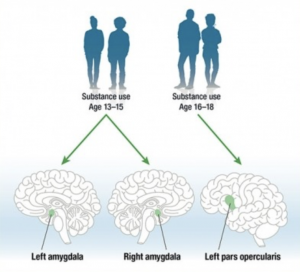
Adolescents who already use gateway drugs have an amplified DeltaFosB accumulation when using other drugs, such as cocaine, to increase the pleasure of the high compared to an adolescent just using a hard drug as cocaine and even compared to adults.
Summation:
Addiction is tough to cope with for the individual, as well as the family and friends of that individual. Addiction treatment is more than just telling someone not taking the drug, but rather a neurological disease that needs more research to be done on effective treatment options. In general, drug education needs to be more extensive on why adolescents should not consume drugs.
Sources:
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2274940/
- https://europepmc.org/article/med/19547960
- https://scholarscompass.vcu.edu/cgi/viewcontent.cgi?article=1491&context=etd
- https://www.routledge.com/rsc/downloads/9781138919105_chapter_2.pdf
- https://europepmc.org/article/med/19547960
- https://www.drugabuse.gov/news-events/nida-notes/2019/04/research-links-adolescent-substance-use-to-adult-brain-volumes
- https://scholarscompass.vcu.edu/cgi/viewcontent.cgi?article=1491&context=etd