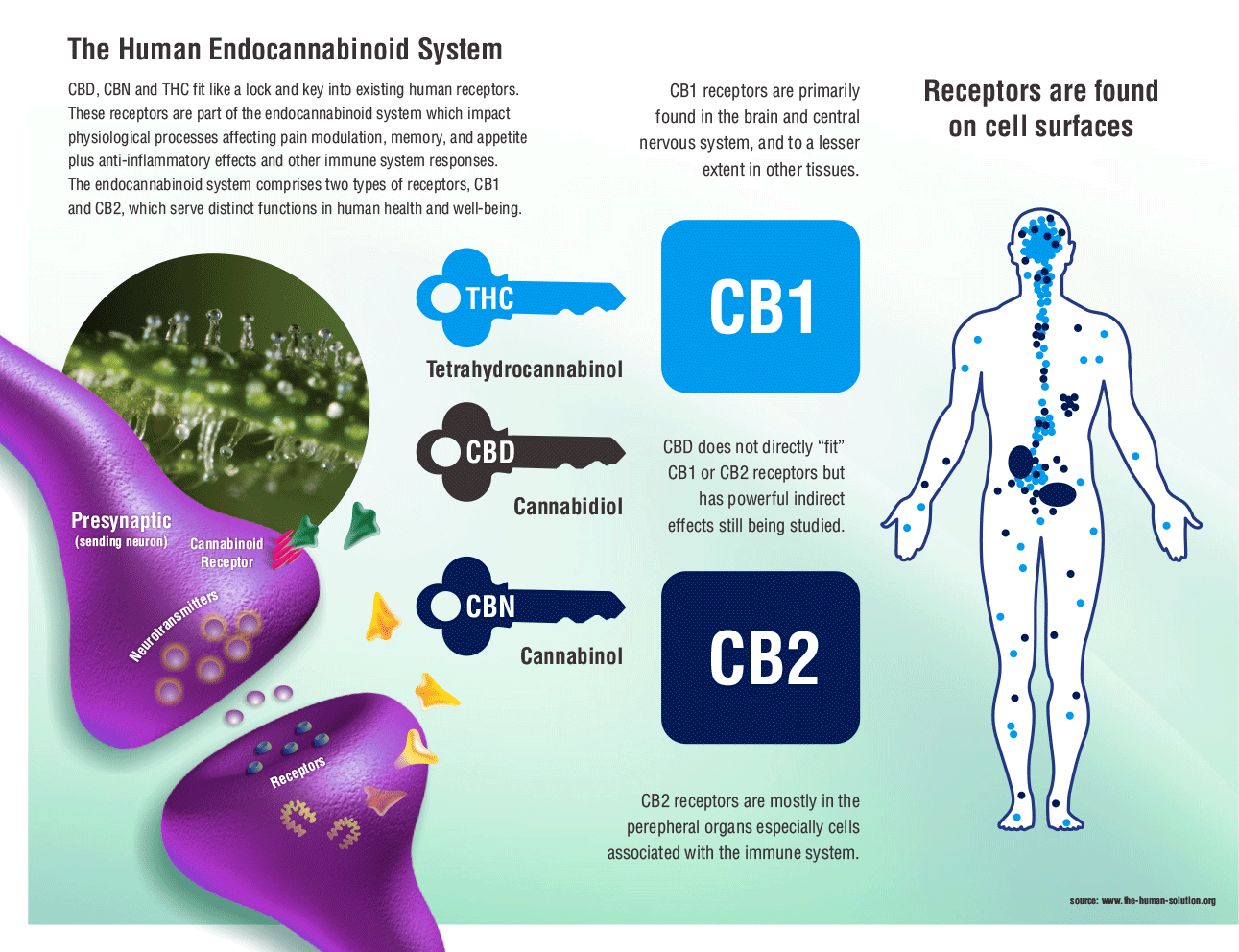
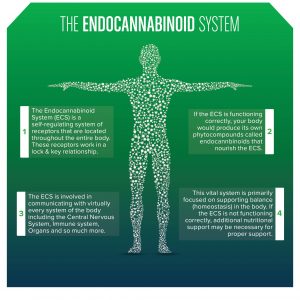
While endocannabinoid’s (eCB) are becoming legalized across the country and even across the world, we still have much to learn about their binding behavior in the brain and in peripheral tissues. Cannabinoids act on almost every tissue of the body, which is why it is used to treat the symptoms of a multitude of diseases such as anxiety, obesity, migraines, chronic pain, and even cancer. The problem with these widespread effects is the inability to narrow down and specify its target action. Maybe you want to reduce chronic pain but not increase appetite, well that isn’t possible with our current endocannabinoid options. The only specificity found thus far is the ability to individually target the cannabinoid receptors CB1 and CB2. CB1 receptors are predominately found in the brain while CB2 receptors are found in immune system tissues and cells. Recent studies have supported the possibility of more receptors but research is limited. To get an understanding of the diversity of endocannabinoid signaling in the body, we will explore some common pathways and therapies.
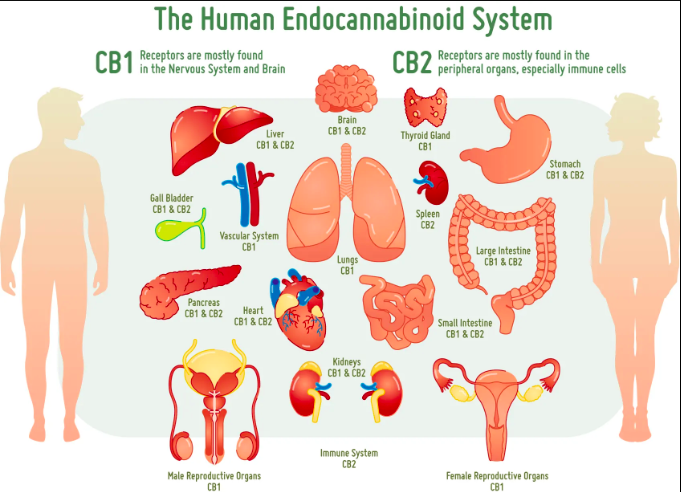
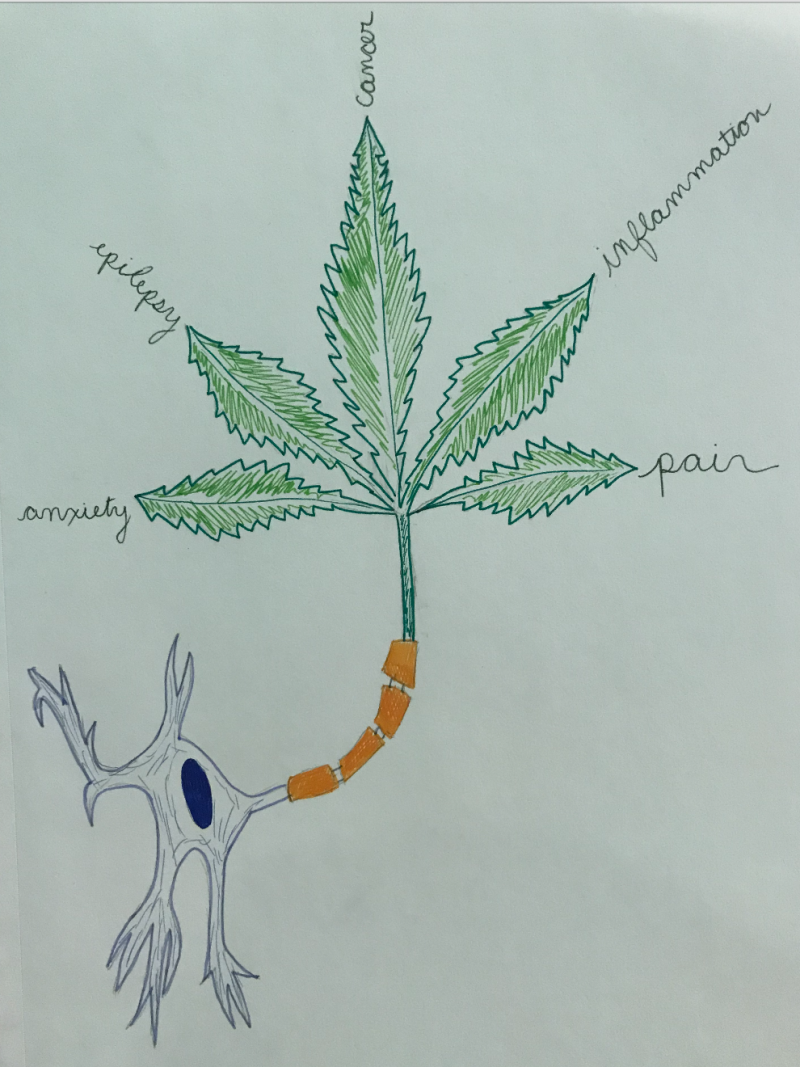
Pain Reception: Endocannabinoids are known to reduce pain, but the mechanism for this effect is opposite of what you would originally expect. TPVR are pain receptors that transduce pain signals to the brain when activated. However, endocannabinoids are known to activate TPVR receptors. The activation of pain receptors can actually cause therapeutic effects by two mechanisms. The first is that eCBs are weak activators of the receptor so if a person is used to really intense pain signaling there will still be a decrease in pain intensity with weak activators instead of strong activators. The second mechanism states that the TPVR receptor is susceptible to desensitization. This means that when activated consistently, the TPVR receptor stops working as effectively leading to decreased pain perception.
Obesity: CB1 receptors in the hypothalamus and nucleus accumbens increase hunger and motivation to eat when activated. Scientists are now trying to see if blocking CB1 receptors can be used as a possible treatment for obesity.
Migraines: An overabundance in nitric oxide (NO) causes inflammation in the brain and eCBs can inhibit NO and also lessen pain perception.
Cancer: Too much eCB activation leads to cell death and cancer cells are known to have an abnormally large amount of CB1 receptors. The increase in receptors is a part of the body’s natural defense mechanism to destroy cancerous cells.
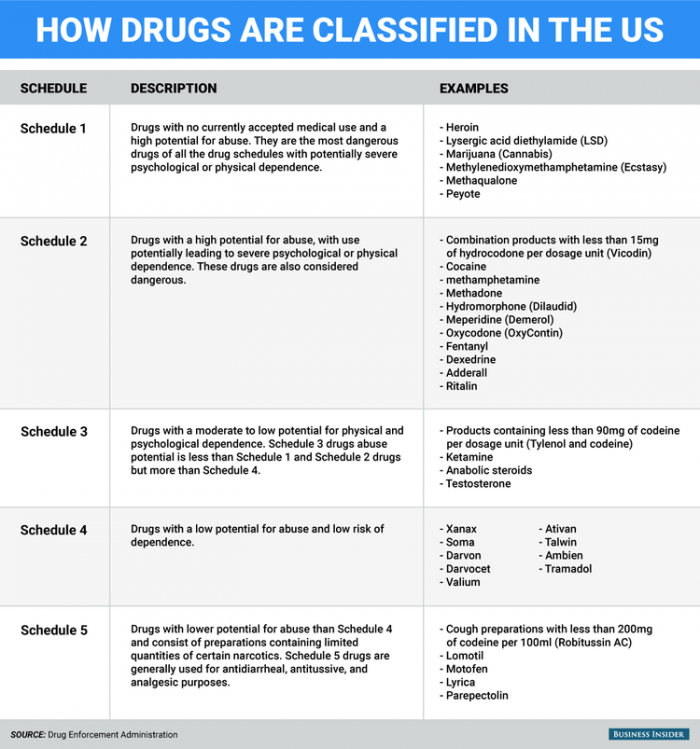
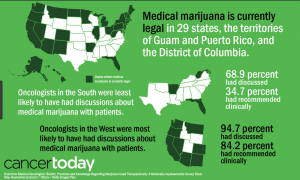
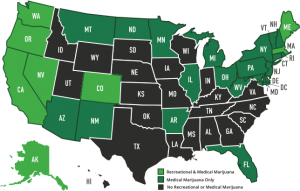
Most of the research above is ongoing because of marijuana’s classification as a schedule one drug. Which means that in order to do research involving marijuana, the facility has to gain DEA approval and increase safety and security protocols which is extremely expensive and time-consuming. This limits our knowledge of marijuana and other endocannabinoid’s signaling behavior. We especially have little data about its biological effects after long-term or chronic use. It would be highly beneficial to know how the body changes to compensate for increased or decreased cannabinoid activity and how to modulate that accordingly. The only way to better prepare our citizens is to reclassify marijuana as a schedule 2 or lower drug so that its research limitations can be reduced. Above is a table of our United States Drug Enforcement Agency’s (DEA) current schedule drug system, which is based on three requirements: 1. That the drug has a high potential for abuse, 2. That the drug has no currently accepted medical treatment inside the U.S. and 3. That there is a lack of accepted safety for use under medical supervision. Obviously, the last two requirements are outdated since medical marijuana has been legalized in 28 states giving the DEA ample evidence to reclassify the drug, but they continue to avoid the subject. Without the reclassification of eCBs, their complexities will never be revealed and further investigation of their therapeutic and harmful effects will continue to evolve at stagnant speeds.

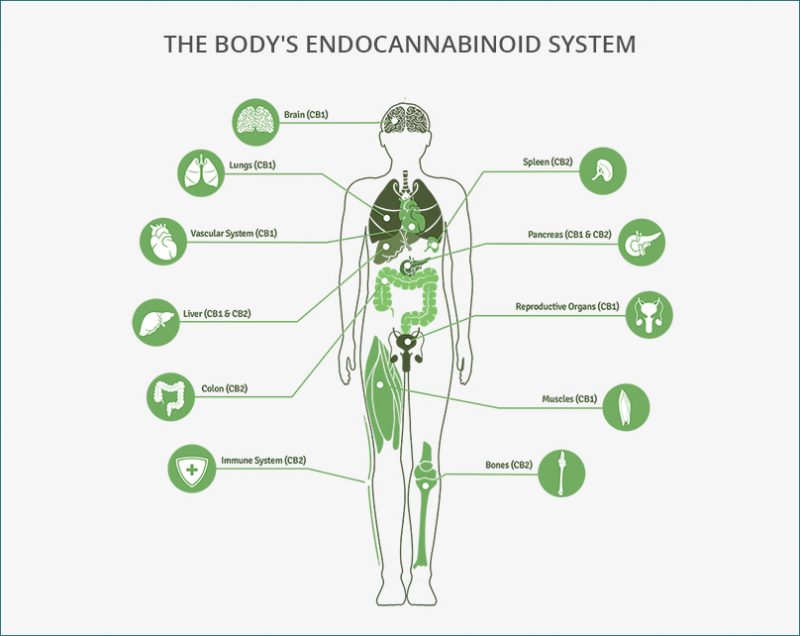
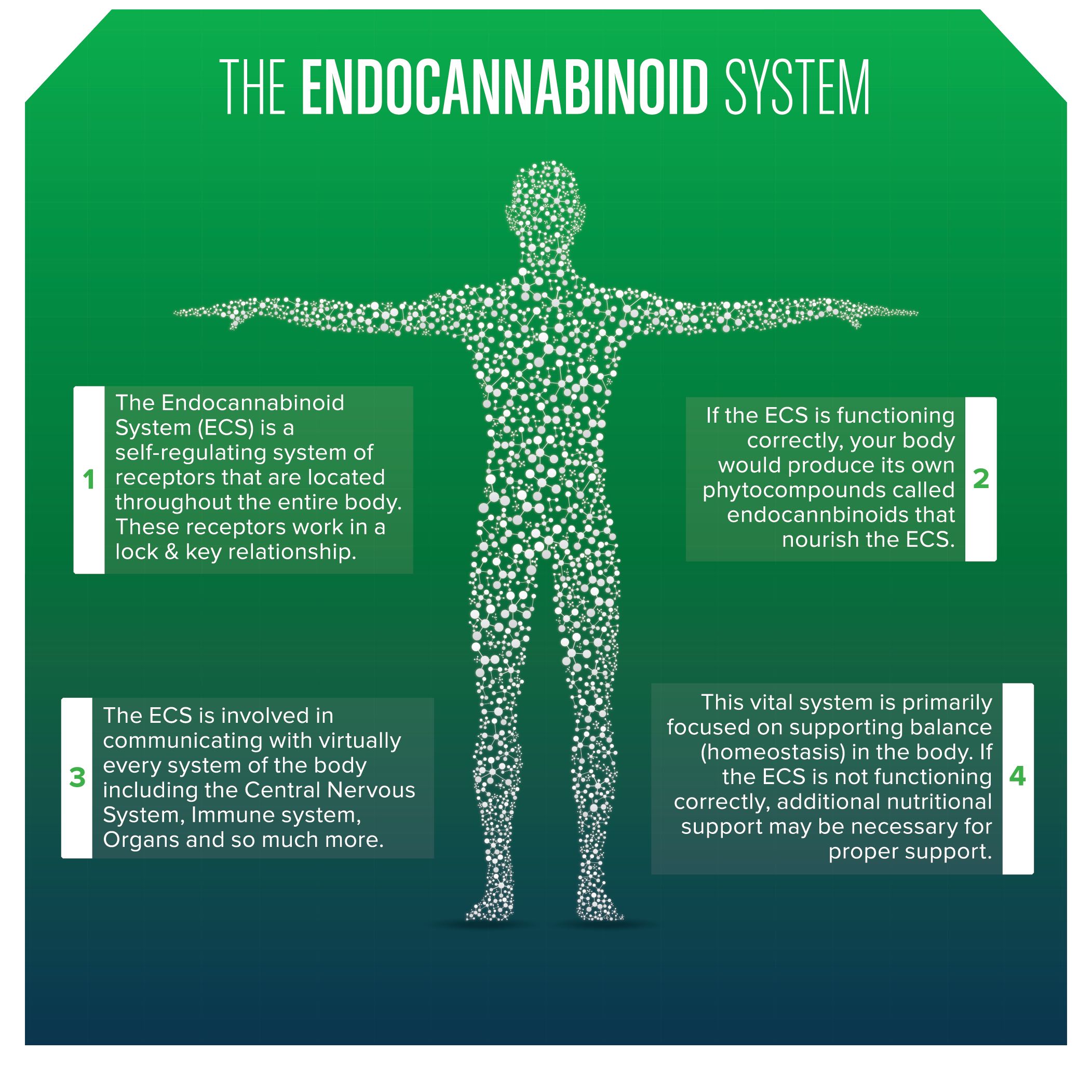
 Due to its classification as a schedule one drug, not enough research has been done on cannabis. Although we don’t understand everything about cannabis, we do know that the endocannabinoid system is present across the human body, not just in the brain. This is one reason why medical marijuana use can aid in pain control, over/under eating, and cancer.
Due to its classification as a schedule one drug, not enough research has been done on cannabis. Although we don’t understand everything about cannabis, we do know that the endocannabinoid system is present across the human body, not just in the brain. This is one reason why medical marijuana use can aid in pain control, over/under eating, and cancer. 






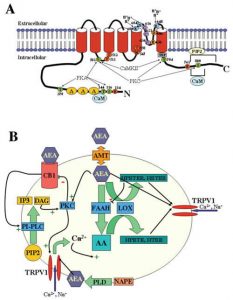





 endocannabinoids. This “on demand” synthesis is done by enzymes that cleave sections of the lipids that go on to signal through the endocannabinoid receptors. The synthesis only takes place when there is an increase in calcium in the cell, membrane depolarization, or receptor activation. With that, endocannabinoid signaling is highly regulated and stabilizes signaling throughout the body.
endocannabinoids. This “on demand” synthesis is done by enzymes that cleave sections of the lipids that go on to signal through the endocannabinoid receptors. The synthesis only takes place when there is an increase in calcium in the cell, membrane depolarization, or receptor activation. With that, endocannabinoid signaling is highly regulated and stabilizes signaling throughout the body.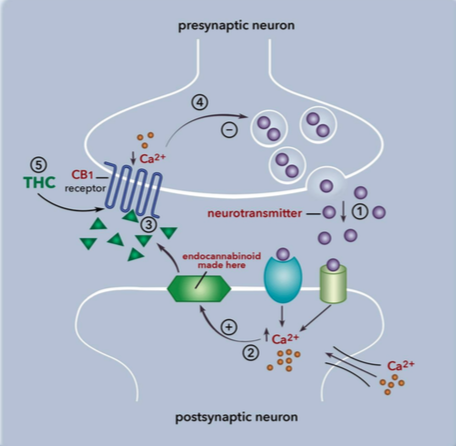 are most often found in the brain and nervous tissue, while CB2 are found in immune tissues. Inhibition of cyclic-AMP leads to a decrease in excitability and signaling, preventing action potentials from spreading between neurons. The endocannabinoid system is a component in many diseases, and can be targeted for many therapeutic benefits. One of these benefits is reducing the signaling associated with pain.
are most often found in the brain and nervous tissue, while CB2 are found in immune tissues. Inhibition of cyclic-AMP leads to a decrease in excitability and signaling, preventing action potentials from spreading between neurons. The endocannabinoid system is a component in many diseases, and can be targeted for many therapeutic benefits. One of these benefits is reducing the signaling associated with pain.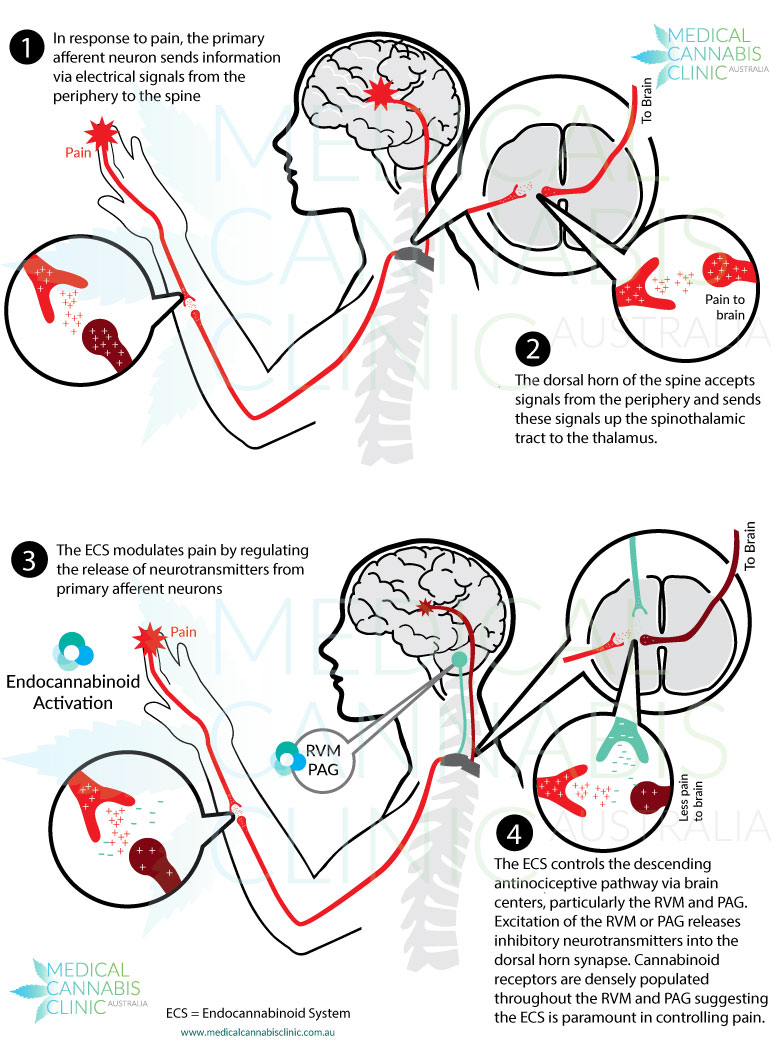 ed to target the pain nociception pathway. Endocannabinoids function to inhibit signaling that uses the excitatory neurotransmitter glutamate by inhibiting cyclic-AMP. In CB1 receptors, this inhibition extends to the pain pathway, and can inhibit signaling in areas of the brain like the amygdala, periaqueductal grey matter, rostral ventromedial medulla, and superficial dorsal horn. CB2 receptors can also play a role in anti-pain signaling, as they are expressed in several cells with inflammatory and immune-competent nature. The inhibition of these cells therefore causes relief from pain caused by inflammation.
ed to target the pain nociception pathway. Endocannabinoids function to inhibit signaling that uses the excitatory neurotransmitter glutamate by inhibiting cyclic-AMP. In CB1 receptors, this inhibition extends to the pain pathway, and can inhibit signaling in areas of the brain like the amygdala, periaqueductal grey matter, rostral ventromedial medulla, and superficial dorsal horn. CB2 receptors can also play a role in anti-pain signaling, as they are expressed in several cells with inflammatory and immune-competent nature. The inhibition of these cells therefore causes relief from pain caused by inflammation.