Abstract created by G. Sparks
Memories play a huge role in our everyday lives. We’re constantly forming them, whether we realize it or not, and they help us navigate the world around us. Built from our experiences and environments, memories allow us to adapt, grow, and prepare for similar situations in the future.1 But not all memories are created equal. Our brains tend to hold onto stressful or traumatic events more strongly, creating lasting imprints that can shape how we think and feel. Over time, these intense memories can contribute to the development of anxiety in certain situations. In more severe cases, they may even lead to conditions like post-traumatic stress disorder (PTSD).
PTSD can seriously impact a person’s quality of life, often bringing nightmares, flashbacks, negative thought patterns, mood swings, and other overwhelming symptoms.1 Many people coping with PTSD or chronic anxiety turn to therapy or medication for relief and those treatments can be incredibly helpful. But what if there were also a natural way to help ease those symptoms? What if something as simple as movement, like exercise that could play a role in healing the brain?
Recent research has been exploring exactly that. Studies show that exercise can act as a form of medicine, helping the brain and body gradually shift out of survival mode and into a healthier, more balanced state.
Understanding the Science Behind Anxiety
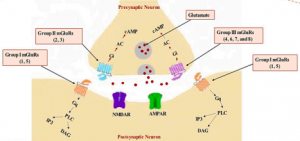
When it comes to the science of anxiety, researchers have proposed many pathways to explain how stress impacts the brain. One particularly interesting pathway involves gene transcription and how it contributes to the consolidation of event-associated memories, especially those tied to stressful or traumatic experiences.1
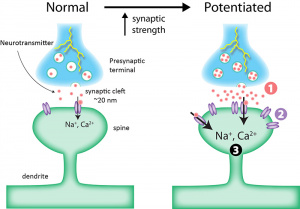
Two key players in this process are corticosterone (a stress hormone) and glutamate (a neurotransmitter). During a stressful event, these molecules work together to set off a cascade of biological events that ultimately encode the memory of that event into long-term storage.
Here’s how it happens: Glutamate binds to NMDA receptors located on dentate gyrus granule neurons in the hippocampus. This binding allows calcium ions to flow into the cell, initiating a signaling pathway that activates MEK, which in turn activates ERK through phosphorylation. ERK then travels into the nucleus of the neuron.
At the same time, corticosterone enters the cell and binds to glucocorticoid receptors (GR), which also move into the nucleus. Once inside, ERK and GR interact, allowing pERK1/2 to phosphorylate two important molecules: MSK1/2 and Elk-1. These molecules then go on to modify the H3 histone by phosphorylating and acetylating it.
This modification causes the chromatin, which was previously tightly wound around the histones and inaccessible, to loosen. This unwinding opens up the DNA, allowing gene transcription to occur. The result is the consolidation of the memory associated with that stressful event.
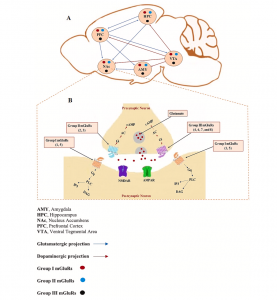
A visual representation of this pathway is shown in Figure 1 below. For a more detailed explanation of the molecular cascade and its components, refer to the full article.

Figure 1. A diagram that illustrates the pathway of corticosterone and glutamate signaling leading to the consolidation of event associated memories. 1
Exercise and Its Role in Reducing Stress and Anxiety
Traditionally, medication and therapy have been the two primary treatments for individuals dealing with anxiety. While both can be highly effective, medications sometimes come with side effects or interactions, especially for people managing other conditions like ADHD.1 In recent years, however, there’s been a growing interest in the therapeutic potential of exercise as a powerful, natural intervention for anxiety.
Research has shown that regular aerobic exercise can reduce the reactivity of both the sympathetic nervous system and the hypothalamic-pituitary-adrenal (HPA) axis which are two key systems involved in the body’s stress response.2 The HPA axis plays a central role in regulating cortisol levels, and exercise has been found to induce long-term adaptations that help moderate stress reactivity and reduce anxiety symptoms.
Exercise also influences several important neurotransmitter systems. In animal studies, physical activity has been linked to increased levels of monoamines such as serotonin, dopamine, and norepinephrine which are chemicals that are commonly targeted by antidepressant medications.2 These changes can produce an antidepressant-like effect and help improve mood stability.
Another important system affected by exercise is the opioid system. Physical activity triggers the release of beta-endorphins, which are natural painkillers and mood enhancers.2 This release is believed to contribute to the well-known “runner’s high” and can significantly lower perceived levels of stress and anxiety.
Additionally, exercise has been shown to increase levels of brain-derived neurotrophic factor (BDNF) which is a key neurotrophin that supports the growth and resilience of neurons.2 Elevated BDNF levels are associated with better emotional regulation, enhanced cognitive function, and improved mental health outcomes.
Together, these biological effects suggest that exercise doesn’t just distract from anxiety, but that it may actually reshape the brain’s response to stress in a meaningful and lasting way.
Here is another link to an article that takes an in depth look on the effects of exercise and physical activity on anxiety.
Final Thoughts
Anxiety is a complex condition that’s closely tied to how our brain stores and responds to stressful events. In some cases, these memories are helpful since they prepare us to react and adapt when similar situations arise in the future. But when the stress is too intense or traumatic, those memories can become harmful. In some individuals, this can lead to the development of PTSD, a condition that often reduces quality of life through persistent fear, anxiety, and emotional distress.
While medications and therapy are effective for many people, they aren’t perfect solutions and can come with complications or limitations. Fortunately, a growing body of research points to exercise as a promising complementary approach. Though it can’t erase trauma, regular physical activity may be a reliable and accessible way to improve mood and reduce the physical and emotional burden of stress.
Whether it’s going for a walk, lifting weights, movement has the power to heal. Exercise truly is a form of medicine, and it might just be one of the most effective tools we have for supporting mental health.
References
(1) Reul, J. M. H. M. Making Memories of Stressful Events: A Journey along Epigenetic, Gene Transcription, and Signaling Pathways. Frontiers in Psychiatry. 2014. https://doi.org/10.3389/fpsyt.2014.00005.
(2) Anderson, E.; Shivakumar, G. Effects of Exercise and Physical Activity on Anxiety. Front Psychiatry 2013, 4 (APR). https://doi.org/10.3389/fpsyt.2013.00027.