Metabolic Syndrome and Obesity
Metabolic Syndrome encompasses several risk factors for heart disease and type 2 diabetes, including high blood pressure, impaired fasting glucose, and obesity. Obesity causes a state of inflammation in the body but can also affect brain regions that regulate energy homeostasis and metabolism. Genetic mutations underlying the development of obesity are almost exclusively found in brain regions that regulate food intake.

Figure 1. Metabolic syndrome and obesity (3).
Melanocortin System
The melanocortin system in the brain is important for the regulation of food intake and energy expenditure (balance), responding to metabolic cues. It operates through the balance between 2 types of neurons: AgRP (orexigenic) and POMC (anorexigenic). When functioning normally, insulin and leptin work in the hypothalamus’s arcuate nucleus (ARC) to activate AgRP neurons when we need to eat and POMC neurons (a-MSH) when we’re full and need to stop eating. AgRP and a-MSH bind to melanocortin receptors (MC4R) in the paraventricular nucleus (PVN) hypothalamic brain region. When inflammation disrupts the melanocortin system, often seen in conditions like obesity and metabolic syndrome, it can disrupt the signals that tell us when to eat and when to stop, leading to overeating and an energy imbalance.
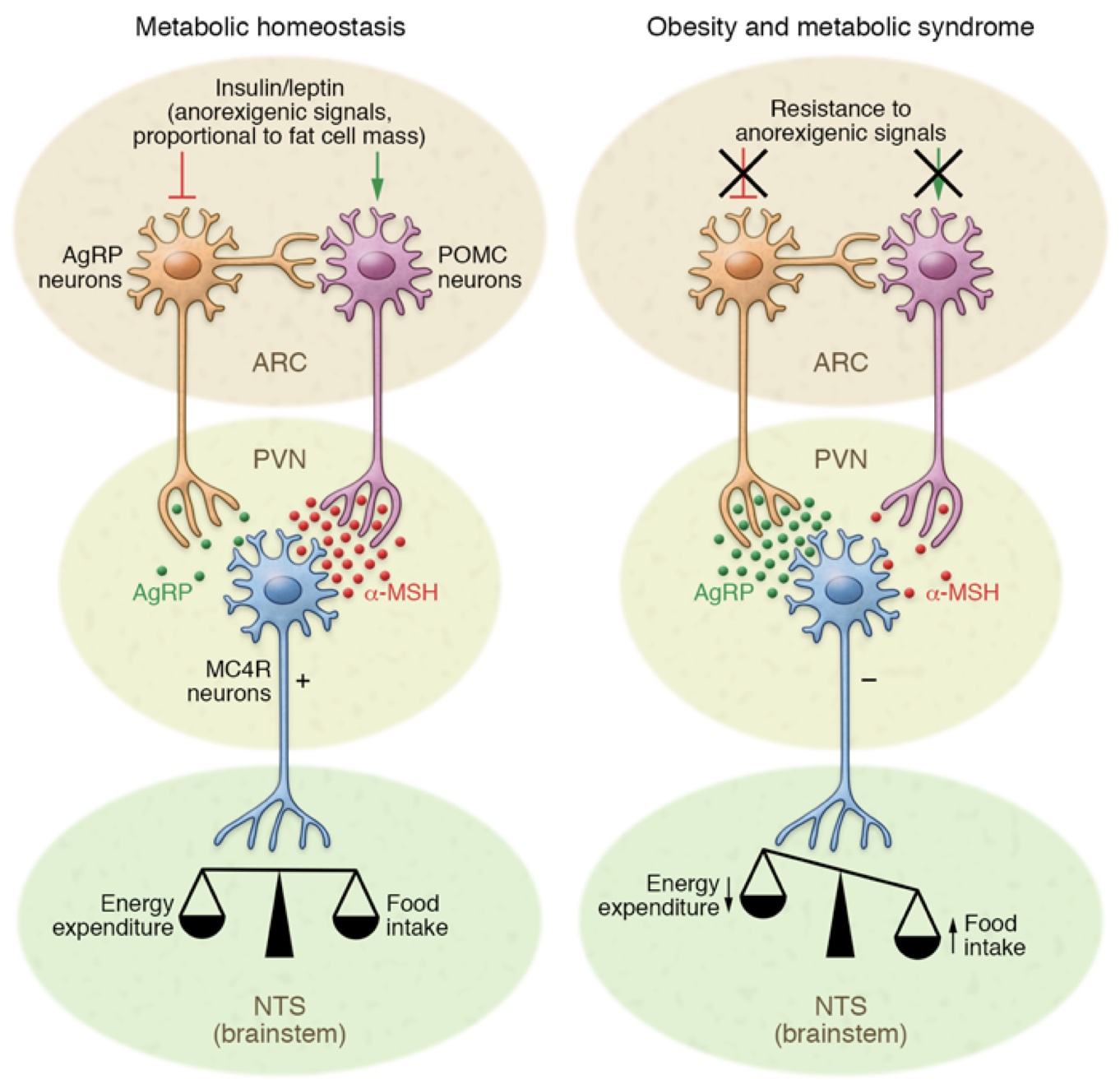
Figure 2. Hypothalamic control of energy homeostasis through the melanocortin system (1).
High-Fat Diets (HFD)
High-fat diets come from the consumption of calorie-dense, high-fat, and high-carbohydrate foods that promote overconsumption and metabolic dysregulation. A high-fat diet elevates the activation of key inflammatory mediators in the hypothalamic pathway like JNK and IKK. The acute effects of HFD feeding are the rapid changes that occur in response to dietary changes. Starting within 3 days, insulin sensitivity in the hypothalamus can decrease, leading to insulin resistance.
Long-chain saturated fatty acids (SFAs) cause resistance to insulin and leptin hormones in how they cross the blood-brain barrier and activate inflammatory pathways (TLR4) that further inhibit insulin and leptin signaling. Apart from TLR4 signaling, the IKK complex keeps NF-kB bound and inactive. When TLR4 becomes active, the IKK and NF-kB separate, and NK-kB becomes active. This activates MAPK signaling and JNK enzymes that inhibit the insulin receptor substrate (IRS) and leads to insulin signaling being inhibited.
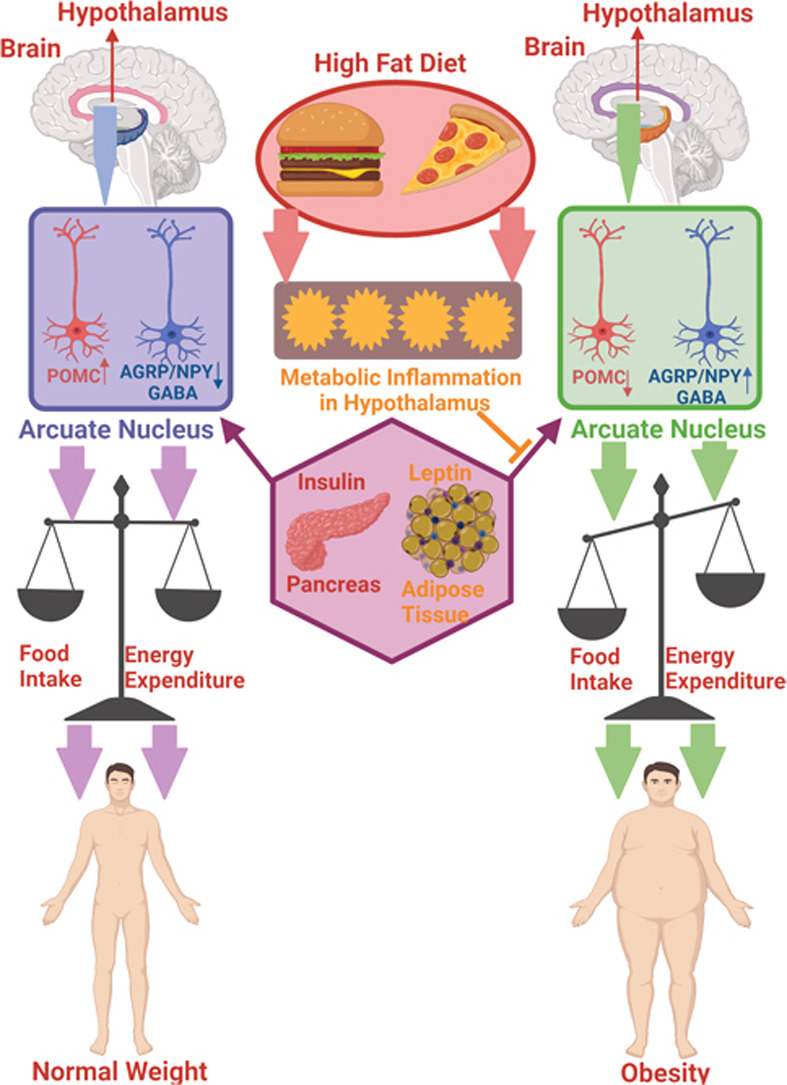
Figure 3. High-fat diets can change your body’s energy balance in a matter of 3 days (4).
Hypothalamic Inflammation
Hypothalamic inflammation from HFD is critical in the development of insulin and leptin resistance, disrupting the body’s ability to regulate glucose metabolism and appetite. As a result, individuals may experience increased food intake, weight gain, and reduced glucose metabolism, contributing to the development of obesity and metabolic dysfunction.
HFD-induced inflammation in the hypothalamus leads to insulin and leptin resistance, eventually causing an imbalance in energy homeostasis (uncoupling of food intake and energy expenditure).
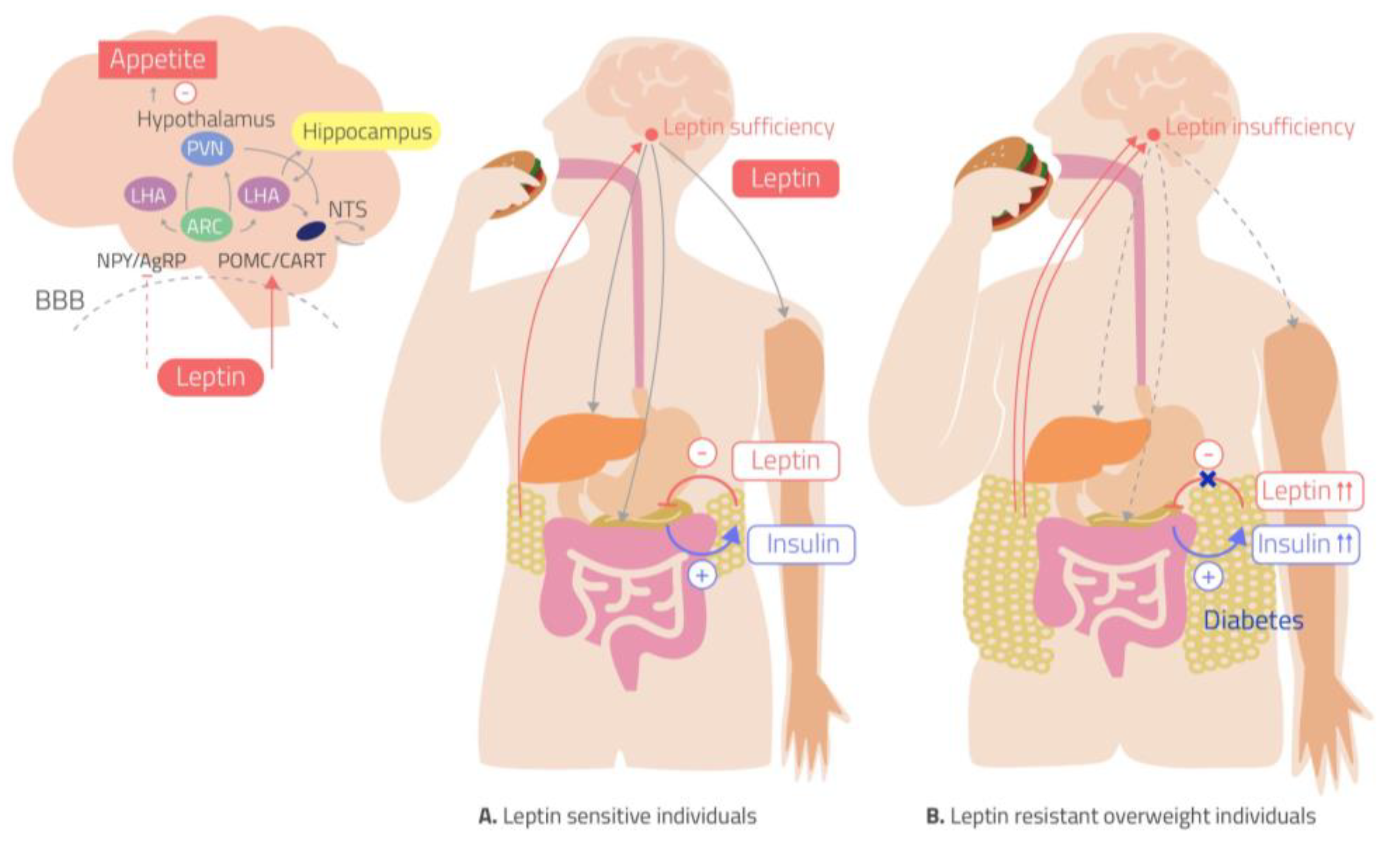
Figure 4. Insulin and leptin resistance in the hypothalamus from HFDs (2).
Microglia and astrocytes are specialized cells in the brain but also contribute to hypothalamic inflammation. Prolonged HFD consumption leads to the accumulation of activated microglia and astrocytes, which produce pro-inflammatory cytokines (TNF-a, IL-6, etc.).
Leaky BBB
Inflammation in the hypothalamus can affect the blood-brain barrier, increasing its permeability. Hypothalamic inflammation triggers the release of pro-inflammatory cytokines and activates glial cells which disrupt the tight junctions between endothelial cells that form and maintain the BBB, leading to increased permeability. This “leak” allows inflammatory mediators to enter the brain, increasing the risk for metabolic dysfunction.
Understanding the neurochemistry of metabolic syndrome, particularly the role of hypothalamic inflammation, offers insights into potential therapeutic targets for addressing obesity, insulin resistance, and related metabolic disorders. By unraveling the complexities of these pathways, researchers aim to develop strategies that restore energy balance and improve metabolic health.

ARTstract created by Kate Loidolt on Canva depicts the pressures of HFDs in today’s society when junk food is everywhere, cheaper, and faster.