
A high fat diet or high caloric intake can activate inflammatory mediators leading to hypothalamic inflammation. Inflammation of the hypothalamus resulting from obesity can affect brain regions that control energy homeostasis and metabolism. This inflammation impairs the energy expenditure and consumption balance resulting in a negative feedback look of overeating and obesity related insulin resistance and inflammation in the brain. There are specific neuronal populations in the brain that express a large number of receptors that bind hormones to be able to respond to cues indicating if you should take in food or expend energy. Because of a high fat diet and the resulting hypothalamic inflammation, the energy expending/ consuming balance is disrupted.
Normal Energy Homeostasis
After eating, AgRP neurons are inhibited when inulin or leptin levels fluctuate due to consuming energy. This causes the activation of POMC neurons to tell the body to stop eating and expend energy. A protein known as FOXO1 is phosphorylated upon insulin signaling and its repression on POMC gene expression is inhibited [1]. Leptin signaling causes STAT3 phosphorylation which induces POMC expression. Melanocyte signaling hormones are released from POMC and act on neurons in the paraventricular nucleus (PVN) of the hypothalamus to diminish food intake [1].
Energy Homeostasis after Eating High Fat Foods
Studies have shown that a high fat diet sustained for at least 3 days in mice can alter insulin and leptin sensitivity in the hypothalamus in rodents. This causes dysfunction with the exergy consuming/ expending feedback loop. Saturated fatty acids specifically are able to cross the blood brain barrier and accumulate in the hypothalamus, leading to inflammation. A high fat diet can also lead to the activation and accumulation of cytokines, which are proinflammatory proteins.
A high fat diet can also activate microglia, astrocytes, and other cell types within the brain. Activated microglia accumulate in the hypothalamus, contributing to inflammation and the production of inflammatory cytokines. Astrocytes maintain synaptic plasticity and aid in cell survival. Following high fat consumption, astrocytes in the hypothalamus release inflammatory signals, also contributing to hypothalamic inflammation. The blood brain barrier can be affected by hypothalamic inflammation due to the decrease in production of tight junction proteins following prolonged exposure to high fat foods. Tanycytes are another cell type that lead to hypothalamic inflammation [1]. They are glial cells that respond to leptin.
TLR4 can be inhibited by pharmacological intervention causing an inhibition of fatty acid induced leptin and insulin resistance. The TLR4 pathway also activates other pathways like the MAPK pathway, triggering the inhibition of insulin action. Another result of high fat diets is the inhibition of the PI3K pathway. Certain downstream affects lead to ER stress and unfolded protein response causing insulin and leptin resistance.
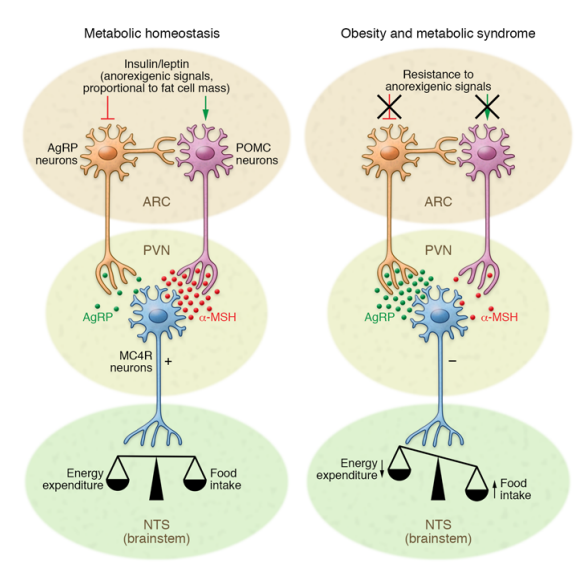
Conclusion
There are a lot of issues that arise from consuming high fat foods including a disruption in the energy expending/ consuming signaling balance and cognitive impairments. Exercises that increase heart rate like running or swimming have reverse effects on hypothalamic inflammation. Being conscious of the food you are eating and being careful of consuming too much fat is a good way to manage hypothalamic inflammation, although it can be hard to truly understand what is in the foods we eat.
References
- Jais, A., & Brüning, J. C. (2017). Hypothalamic inflammation in obesity and metabolic disease. Journal of Clinical Investigation, 127(1), 24–32. https://doi.org/10.1172/JCI88878