Artstract: healthy foods = healthy brain.
Obesity is a rising problem for the global population, and can give rise to a variety of other health issues, such as metabolic syndrome and its aftereffects. This rise comes from new eating patterns occurring from a high-fat and calorie dense foods, in combination with physical activity levels, etc. Through different pathways obesity causes changes in metabolism and has been linked to hypothalamic inflammation. And it was found that diet induced brain inflammation leads to uncoupling of food intake and energy expenditure[1].
What is metabolic syndrome?
Metabolic syndrome is an accumulation of several disorders, that togher raise a risk of developing a number of diseases and health conditions such as cardiovascular disease, insulin resistance, and diabetes type 2, additionally developing vascular and neurological complications[2]. It is believed to affect about 25% of adults worldwide[3].
There is various contributors to the development of metabolic syndrome, from genetics, to life style and environmental factors such as overeating and lack of physical activity. The contributors further contribute to mechanisms proposed to play a further role in the development of metabolic syndrome, as well as the progression to subsequent disorders (cardiovascular disease and diabetes type 2), these are insulin resistance, chronic inflammation, and neurohormonal activation[4].
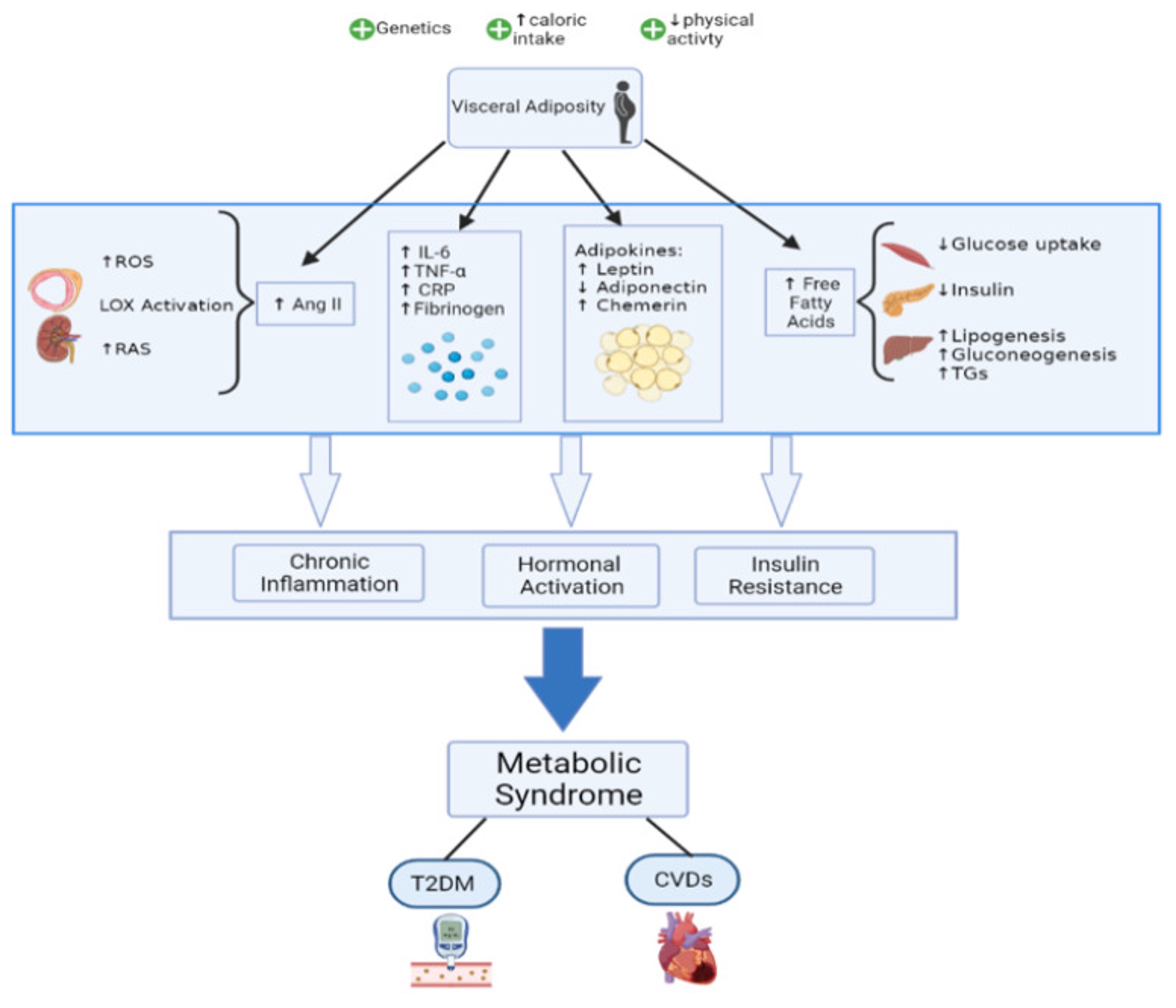
Figure 1: The route of development of metabolic syndrome, to futher progression to diseases[5].
Hypothalamic inflammation
Hypothalamic inflammation has been linked to the development and progression of obesity, and it impacts various mechanisms, such as energy balance, insulin resistance, and alterations in the blood-brain barrier (BBB).
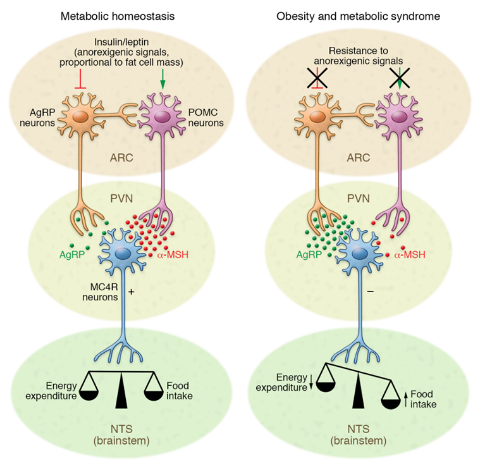
Figure 2: Hypothalamic control of energy homeostasis[6].
The hypothalamus controls a number of functions that incorporate metabolic feedback and that regulates energy homeostasis. Balancing energy intake and energy expenditure is energy homeostasis. This energy homeostasis happens when the body is exposed to an increased energy intake and decreased energy expenditure that causes inhibition of POMC activation and activates AgRP neurons by inhibiting insulin and leptin, which is shown in Figure 1. Insulin and Leptin are important to control your bodies regulation to tell you when you are full, and when they are inhibited that messes with this regulation. The inhibition affects POMC which is a anorexigenic peptide that tells the body to not eat, while the activation of AgRP that is an orexigenic peptide oppositely tells the body to eat. The inflammatory process is a two step process divided into an early inflammatory phase, and a secondary prolonged inflammatory phase that is sustained by the exposure to a high-fat diet (HFD) which leads to the activation of cellular stress mechanisms[7].
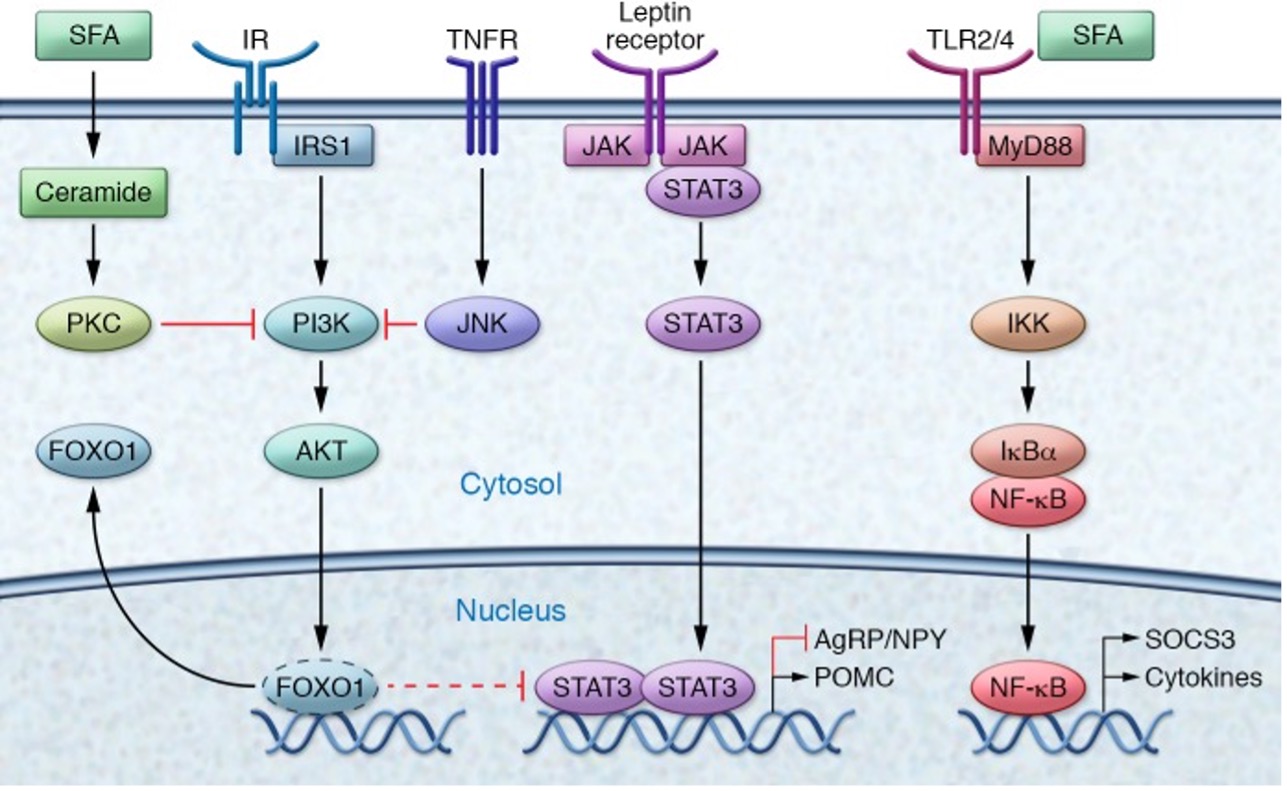
Figure 3: The molecular pathway of hypothalamic inflammation[8].
As pictured in Figure 2, the different inhibitors of insulin and leptin pathways, that alterates transcription. JNK from TNFR and PKC from saturated fatty acids (SFA) inhibits the the Insulin (PI3K pathway), and inhibits Akt. That leads FOXO1 to inhibit JAK/STAT signaling, and it activates AgRP and inhibits POMC, which leads us to eat more or keep eating. However, when Akt is active it keeps FOXO1 out of the nucleus.
In the matter of the blood-brain barrier (BBB), there has been found alterations that are involved in the development of hypothalamic inflammation. These alterations concern permeability and disruption of the BBBs integrity, which has been found to be caused by long-term HFD (through VEGF expression in astrocytes and tanycytes in the hypothalamus)[9].
Acute and Chronic Effects
The exposure to HFD has acute effects on the brain, it was found in rodents that already after three days it was found to significantly red ce hypothalamic insulin sensitivity[10]. And was found to upregulate SOCS3 in AgRP neurons, which lead to the effects on previous mentioned mechanisms such as energy imbalance and leptin and insulin resistance. Chronically, inflammatory mediators can give rise to long-lasting impaired metabolic control of the hypothalamus, that leads to apoptosis of neurons and a reduction of synaptic inputs[11].
Bibliography
[1] Jais, A., & Brüning, J. C. (2017). Hypothalamic inflammation in obesity and metabolic disease. The Journal of clinical investigation, 127(1), 24–32. https://doi.org/10.1172/JCI88878
[2] Swarup S, Goyal A, Grigorova Y, et al. Metabolic Syndrome. [Updated 2022 Oct 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459248/
[3] Rojas, M., Chávez-Castillo, M., Pirela, D., Parra, H., Nava, M., Chacín, M., Angarita, L., Añez, R., Salazar, J., Ortiz, R., Durán Agüero, S., Gravini-Donado, M., Bermúdez, V., & Díaz-Camargo, E. (2021). Metabolic Syndrome: Is It Time to Add the Central Nervous System?. Nutrients, 13(7), 2254. https://doi.org/10.3390/nu13072254
[4] Fahed, G., Aoun, L., Bou Zerdan, M., Allam, S., Bou Zerdan, M., Bouferraa, Y., & Assi, H. I. (2022). Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. International journal of molecular sciences, 23(2), 786. https://doi.org/10.3390/ijms23020786
[5] Ibid.
[6] Jais, A., & Brüning, J. C. (2017). Hypothalamic inflammation in obesity and metabolic disease. The Journal of clinical investigation, 127(1), 24–32. https://doi.org/10.1172/JCI88878
[7] Ibid.
[8] Ibid.
[9] Ibid.
[10] Ibid.
[11] Ibid.